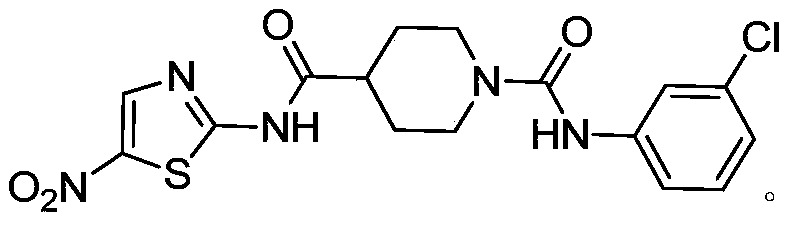
A novel thiazole compound xqh-3-6 against Streptococcus mutans and its application
A technology of streptococcus mutans and compounds, applied in the field of thiazole compounds and new thiazole compounds, to achieve small molecular weight, good killing effect and strong inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of compound XQH-3-6
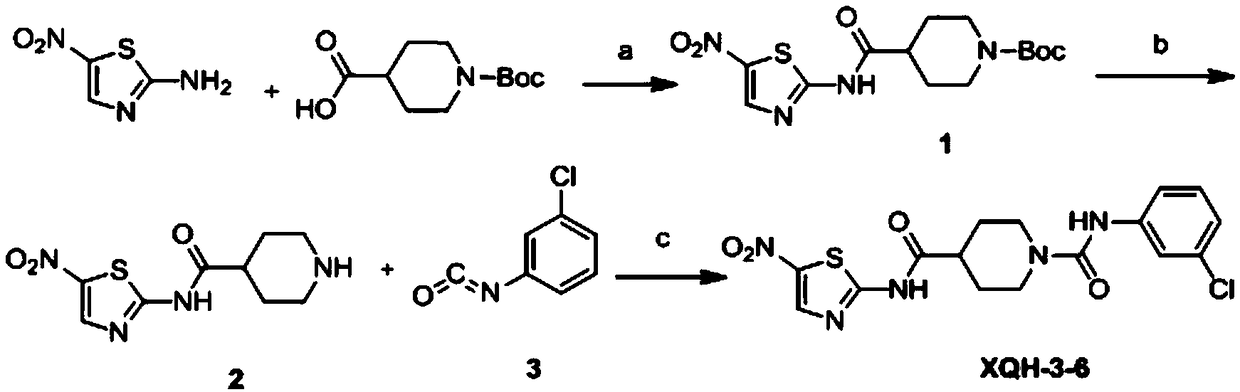
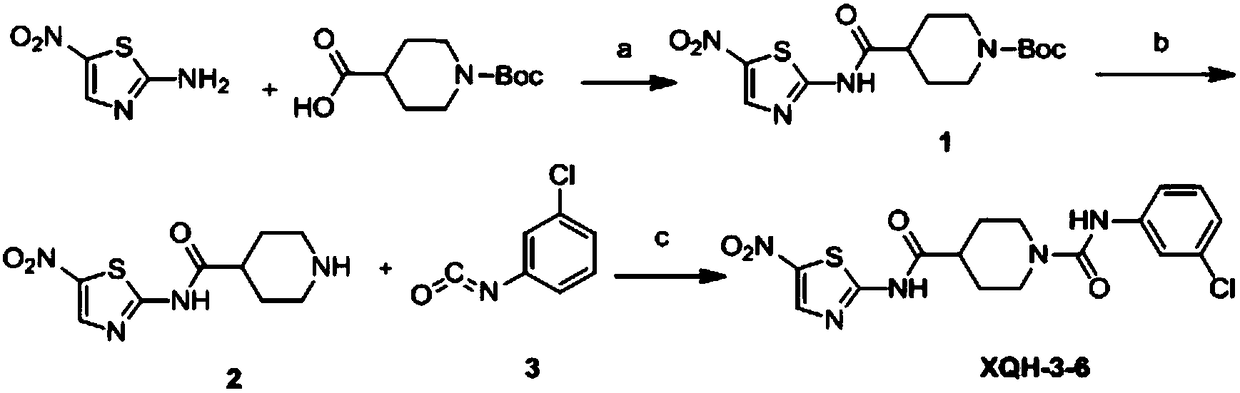
[0024] The synthetic route of compound XQH-3-6 is shown in the following reaction formula:
[0025]
[0026] Wherein: (a) 1-hydroxybenzotriazole (HOBT), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDCI), triethylamine, room temperature; ( b) ethyl acetate saturated hydrogen chloride solution, room temperature; or trifluoroacetic acid / dichloromethane; (d) triphosgene, anhydrous ethyl acetate, 0°C to reflux; (e) triethylamine, anhydrous tetrahydrofuran, 0°C to room temperature.
[0027] If the solvent involved in the reaction process needs to be dried, it is dried according to a commonly used standard drying method. Specific reaction process:
[0028] (1) Preparation of intermediate 4-tert-butyl-((5-nitrothiazol-2-yl)carbamoyl-1-carboxylic acid (1).
[0029] Dissolve 1-Boc-4-piperidinecarboxylic acid (1eq) in N, N dimethylformamide, then add HOBt (1.2eq) and EDCI (1.2eq) respectively, add triethyl...
Embodiment 2
[0035] Embodiment 2: the preparation of Streptococcus mutans
[0036] (1) The medium for cultivating Streptococcus mutans is Brain Heart Infusion medium (brand OXOID, product number CM1135). The main components of the medium are Brain infusion solids 12.5g / L, Beef heartinfusion solids 5.0g / L , Proteose peptone 10.0g / L, Glucose 2.0g / L, Sodiumchloride 5.0g / L, Di-sodium phosphate2.5g / L, pH 7.4±0.2. If solid medium is required, add 1.5% agar powder. Sterilize with damp heat at 115°C for 30 minutes, and cool down for use.
[0037] (2) The culture medium for Streptococcus mutans biofilm is brain heart infusion-sucrose medium. The preparation method is that the sucrose is made into 20% stock solution and sterilized by filtering with a 0.22 μm sterile filter, and the final concentration of 1% sucrose is added to the brain-heart infusion medium.
[0038] (3) Streptococcus mutans type strain UA159 and clinical strain UA246 were inoculated on the solid medium of brain heart infusion a...
Embodiment 3
[0042] Example 3: Activity detection of compound XQH-2-92 on streptococcus mutans planktonic cells
[0043] (1) Prepare Streptococcus mutans liquid and compound XQH-3-6 according to the method described in embodiment 2, will cultivate logarithmic phase (OD 600 =0.8~1.0) Streptococcus mutans UA159 and UA246 bacterium liquid were diluted with brain heart infusion liquid medium to a final concentration of 5×10 5 cfu / ml for use.
[0044] (2) The detection of the minimum inhibitory concentration of compound XQH-3-6 on planktonic cells of Streptococcus mutans UA159 and UA246 was carried out by micro broth dilution method. The final concentration of Streptococcus mutans in each well of a sterile 96-well plate was 5×10 5 cfu / ml, add the stock solution of compound XQH-3-6 prepared according to the above method to the first well and adjust to a final concentration of 256 mg / L, mix well, then pipette 150 μl to the second well, mix well and then pipette 150 μl To the 3rd well, serially...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com