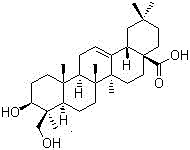
Pharmaceutical composition for treating optic atrophy
A technology of optic atrophy and composition, which is applied in the direction of drug combination, nervous system diseases, active ingredients of heterocyclic compounds, etc., and can solve problems such as optic atrophy and loss of function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: The pharmaceutical composition and preparation method thereof for the treatment of optic atrophy
[0038] The composition and parts by weight of the crude drug of the pharmaceutical composition for treating optic atrophy are: 484g of Sanzuanfeng, 266g of Herbal Triangle Maple, 45g of Asarin, 35g of Genistin, and 9g of Hederagenin;
[0039] Preparation:
[0040] (1) According to the ratio of raw materials, take Sanzuanfeng, herbal triangular maple, asarin, genistin, and hedera saponin, mix them evenly, use ethanol with a concentration of 42% by weight as a solvent, and extract by warm soaking at 32°C. The number of extractions is 9 times, each extraction time is 38 hours, and the amount of solvent used each time is 37 times the total weight of the raw material drug, filtered to obtain medicinal residue A and extract A, extract A reclaims ethanol, and concentrates to a relative density of 1.06 , filtered, the medicinal solution passes through the HPD750 mac...
Embodiment 2
[0043] Embodiment 2: the pharmaceutical composition and preparation method thereof for the treatment of optic atrophy
[0044] The composition and parts by weight of the crude drug of the pharmaceutical composition for treating optic atrophy are: 481g of Sanzuanfeng, 268g of Herbal Triangle Maple, 42g of Asarin, 36g of Genistin, and 8g of Hederagenin;
[0045] Preparation:
[0046] (1) According to the ratio of raw materials, take Sanzuanfeng, herbal triangular maple, asarin, genistin, and hedera saponin, mix them evenly, use ethanol with a concentration of 42% by weight as a solvent, and extract by warm soaking at 32°C. The number of extractions is 9 times, each extraction time is 38 hours, and the amount of solvent used each time is 37 times the total weight of the raw material drug, filtered to obtain medicinal residue A and extract A, extract A reclaims ethanol, and concentrates to a relative density of 1.06 , filtered, the medicinal solution passes through the HPD750 mac...
Embodiment 3
[0049] Embodiment 3: the pharmaceutical composition and preparation method thereof for the treatment of optic atrophy
[0050] The composition and parts by weight of the crude drug of the pharmaceutical composition for treating optic atrophy are: 487g of Sanzuanfeng, 264g of Herbal Triangle Maple, 48g of Asarin, 34g of Genistin, and 10g of Hederagenin;
[0051] Preparation:
[0052] (1) According to the ratio of raw materials, take Sanzuanfeng, herbal triangular maple, asarin, genistin, and hedera saponin, mix them evenly, use ethanol with a concentration of 42% by weight as a solvent, and extract by warm soaking at 32°C. The number of extractions is 9 times, each extraction time is 38 hours, and the amount of solvent used each time is 37 times the total weight of the raw material drug, filtered to obtain medicinal residue A and extract A, extract A reclaims ethanol, and concentrates to a relative density of 1.06 , filtered, the medicinal solution passes through the HPD750 ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com