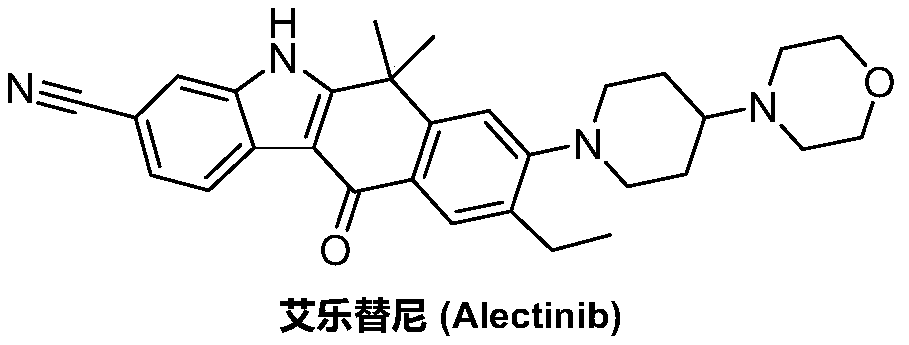
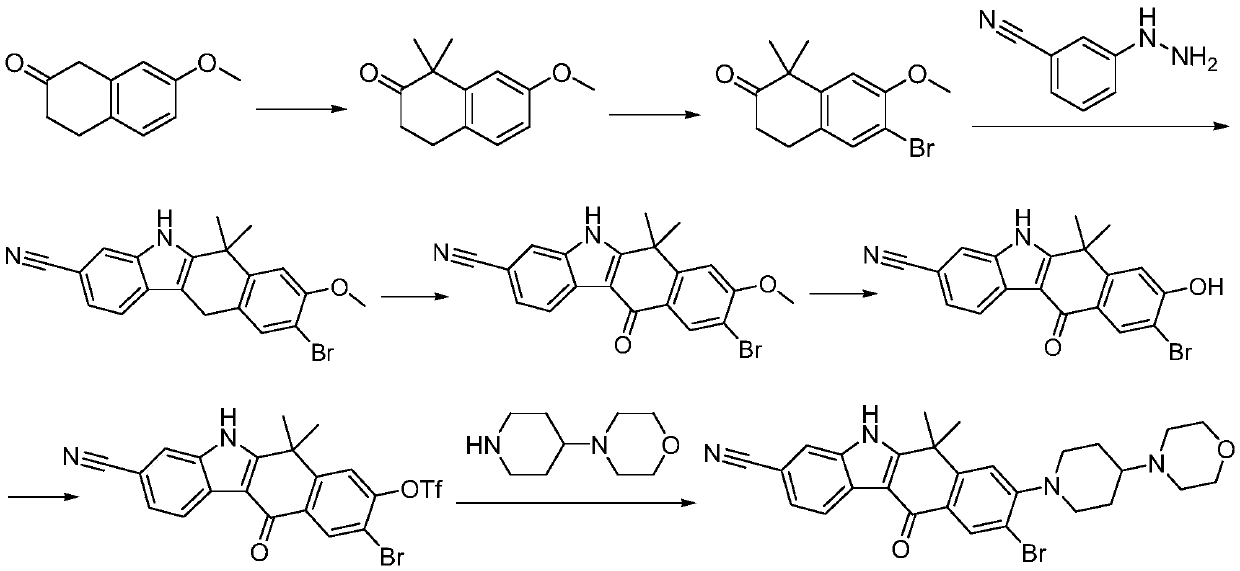
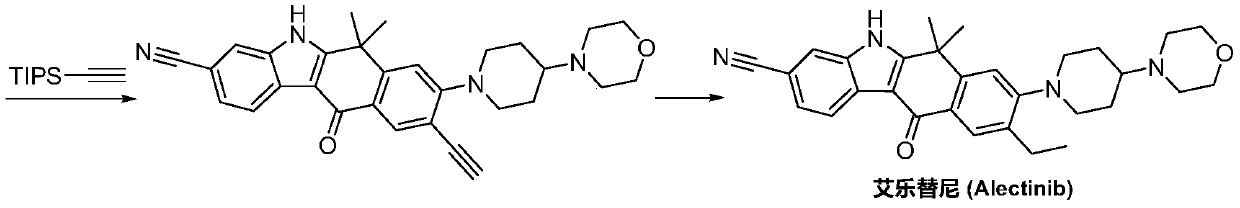
A kind of preparation method of alectinib
A technology of alectinib and ethyl, which is applied in the field of pharmaceutical chemical synthesis, can solve the problems of unfavorable industrial production promotion, expensive starting materials, and the use of a large amount of solvents, and achieves easy and effective control of reaction conditions, less impurities, and easy reagents. The effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A) Preparation of 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoic acid tert-butyl ester:
[0033] 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (10.0g, 24mmol) was dissolved in N,N-dimethylformamide (200mL), 4-(4-piperidinyl)morpholine (9.2g, 54mmol) and sodium methoxide (3.2g, 59mmol) were added, the reaction mixture was stirred at 100°C for 12 hours, the reaction solution was cooled to room temperature, water (40mL) was added, and cooled Crystallize at -10°C for 3 hours and filter to obtain 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3 -Tert-Butyl oxovalerate, white solid (9.6g), yield 87%.
[0034] B) Preparation of 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H -Indole-3-carboxylic acid:
[0035] 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate tert-butyl ester (9.0g, 20mmol), 3-cyanophenylhydrazine (3.3g, 25mmol) and trifluoroaceti...
Embodiment 2
[0039] A) Preparation of 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoic acid tert-butyl ester:
[0040] 4-(4-Ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (5.0g, 12mmol) was dissolved in toluene (100mL), and 4-(4-piperidine was added Yl)morpholine (5.5g, 32mmol), sodium ethoxide (2.4g, 35mmol), the reaction mixture was stirred at 110°C for 6 hours, the reaction solution was cooled to room temperature, water (50mL) was added, and cooled to -10°C for 4 hours to crystallize , Filter to obtain 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate tert-butyl ester , White solid (5.0g), yield 91%.
[0041] B) Preparation of 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H -Indole-3-carboxylic acid:
[0042] 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoate tert-butyl ester (5.0g, 11mmol), 3-cyanophenylhydrazine (2.0g, 15mmol) and acetic acid (45.8g, ...
Embodiment 3
[0046] A) Preparation of 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoic acid tert-butyl ester:
[0047] 4-(4-ethyl-3-iodophenyl)-4-methyl-3-oxopentanoic acid tert-butyl ester (13.5g, 32mmol) was dissolved in 1,4-dioxane (250mL) and added 4-(4-piperidinyl)morpholine (10.0g, 59mmol), sodium tert-butoxide (6.2g, 65mmol), the reaction mixture was stirred at 90°C for 18 hours, the reaction solution was cooled to room temperature, and water (120mL) was added. Cool to -5°C and crystallize for 4 hours and filter to obtain 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl- Tert-Butyl 3-oxopentanoate, white solid (12.6 g), yield 86%.
[0048] B) Preparation of 6-cyano-2-{2-[4-ethyl-3-(4-(morpholin-4-yl)piperidin-1-yl)phenyl]prop-2-yl}-1H -Indole-3-carboxylic acid:
[0049] 4-{4-ethyl-3-[4-(morpholin-4-yl)piperidin-1-yl]phenyl}-4-methyl-3-oxopentanoic acid tert-butyl ester (12.5g, 27mmol), 3-cyanophenylhydrazine (4.0g, 30mmol) and formic acid (5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com