A kind of preparation method for the hydrogen production catalyst of steam reforming of methane
A technology for producing hydrogen from methane steam and reforming, which is applied in catalyst activation/preparation, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc. High problems, to achieve the effect of being conducive to industrial amplification, reducing metal consumption, and reducing concentration difference
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
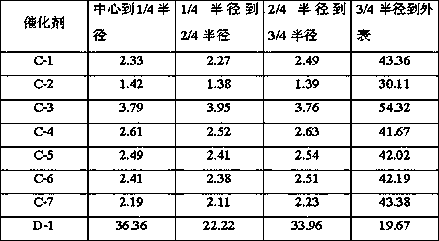
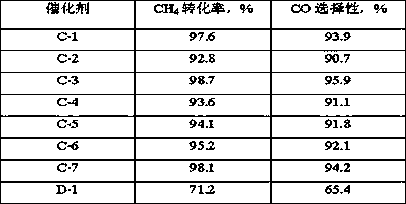
Image
Examples
Embodiment 1
[0023] The spent hydrotreating catalyst (MoCo / Al 2 o 3), the oil on the catalyst surface was removed by petroleum ether extraction, dried at 110°C for 8 h, and the obtained catalyst was calcined at 450°C for 4 h to obtain catalyst precursor A, wherein Mo accounted for 6.1wt% by weight of catalyst precursor A in terms of elements, Co accounts for 1.8wt% of the weight of the catalyst precursor A in terms of elements, and Ni accounts for 2.3wt% of the weight of the catalyst precursor A in terms of elements; 20g of the catalyst precursor A is activated in a hydrogen-containing mixed atmosphere, and the volume content of hydrogen in the mixed gas is 80%, the reduction condition is 450°C, 0.2MPa (absolute pressure), and the reduction time is 4h; dissolve 19.02g of magnesium nitrate in 30mL of deionized water to obtain solution B, and mix it with 4 times the mass of a 40% furfural aqueous solution Mix evenly, and then add it to the autoclave together with the reduced and activated c...
Embodiment 2
[0025] The spent hydrotreating catalyst (MoCo / Al 2 o 3 ), the oil on the catalyst surface was removed by petroleum ether extraction, dried at 110°C for 8 h, and the obtained catalyst was calcined at 450°C for 4 h to obtain catalyst precursor A, wherein Mo accounted for 5.2wt% by weight of catalyst precursor A in terms of elements, Co accounts for 1.2wt% of the weight of the catalyst precursor A in terms of elements, and Ni accounts for 1.5wt% of the weight of the catalyst precursor A in terms of elements; 20g of the catalyst precursor A is activated in a hydrogen-containing mixed atmosphere, and the volume content of hydrogen in the mixed gas is 80%, the reduction condition is 450°C, 0.2MPa (absolute pressure), and the reduction time is 4h; dissolve 10.26g of magnesium nitrate in 25mL of deionized water to obtain solution B, and mix it with 4 times the mass of a 40% furfural aqueous solution Mix evenly, and then add it to the autoclave together with the reduced and activated ...
Embodiment 3
[0027] The spent hydrotreating catalyst (MoCo / Al 2 o 3 ), the oil on the catalyst surface was removed by petroleum ether extraction, dried at 110°C for 8 h, and the obtained catalyst was calcined at 450°C for 4 h to obtain catalyst precursor A, wherein Mo accounted for 6.1wt% by weight of catalyst precursor A in terms of elements, Co accounts for 1.8wt% of the weight of the catalyst precursor A in terms of elements, and Ni accounts for 2.3wt% of the weight of the catalyst precursor A in terms of elements; 20g of the catalyst precursor A is activated in a hydrogen-containing mixed atmosphere, and the volume content of hydrogen in the mixed gas is 80%, the reduction condition is 450°C, 0.2MPa (absolute pressure), and the reduction time is 4h; 27.99g of magnesium nitrate is dissolved in 40mL of deionized water to obtain solution B, and its mass fraction is 4 times the mass fraction of 40% furfural aqueous solution Mix evenly, and then add it to the autoclave together with the re...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com