An iron-based coordination compound with antitumor activity
A technology of coordination compounds and anti-tumor activity, which is applied to iron group organic compounds without C-metal bonds, iron organic compounds, anti-tumor drugs, etc., to achieve good in vitro inhibitory activity and simple synthesis method.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0018] In order to make the content of the present invention easier to understand, the technical solutions of the present invention will be further described below in conjunction with specific embodiments, but the present invention is not limited thereto.
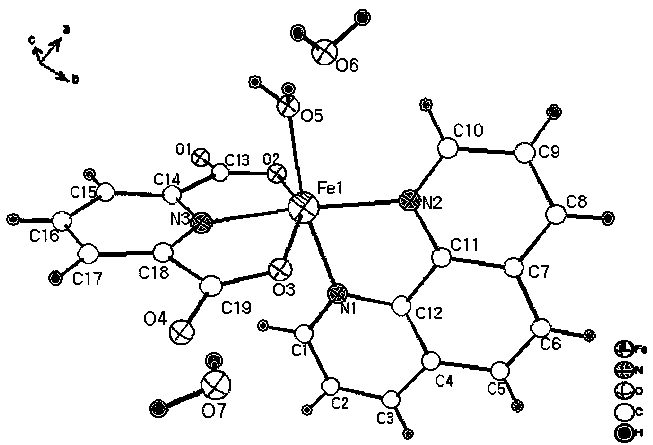
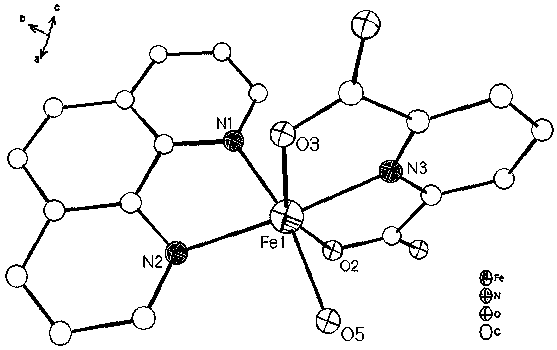
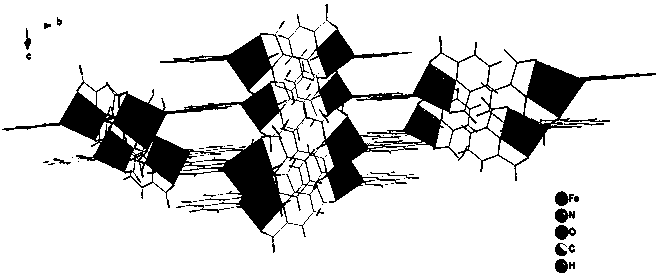
[0019] Put 2.05 mmol 2,6-pyridinedicarboxylic acid and 1.2 mmol 1,10-phenanthroline into a beaker, add 15 mL deionized water, stir and mix for 15 min, then add 1.2 mmol FeCl 3 •6H 2 O solid, continue stirring at room temperature for 3 hours to obtain a reddish-brown clear solution, filter the solution, and the filtrate is left to evaporate at room temperature for 2 weeks, and then reddish-brown massive crystals are precipitated, and then filtered and dried to obtain the iron-based coordination compound [Fe (C 7 h 3 NO 4 )(C 12 h 8 N 2 ) • H 2 O]•2H 2 O single crystal with a yield of 65% (calculated as Fe).
[0020] 1. [Fe(C 7 h 3 NO 4 )(C 12 h 8 N 2 ) • H 2 O]•2H 2 Characterization of O single crystals
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com