Application of fluoro-thiosemicarbazone compound in antitumor drug
An anti-tumor drug, thiosemicarbazide technology, applied in the field of medicine, can solve the problem of no fluorinated thiosemicarbazide compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
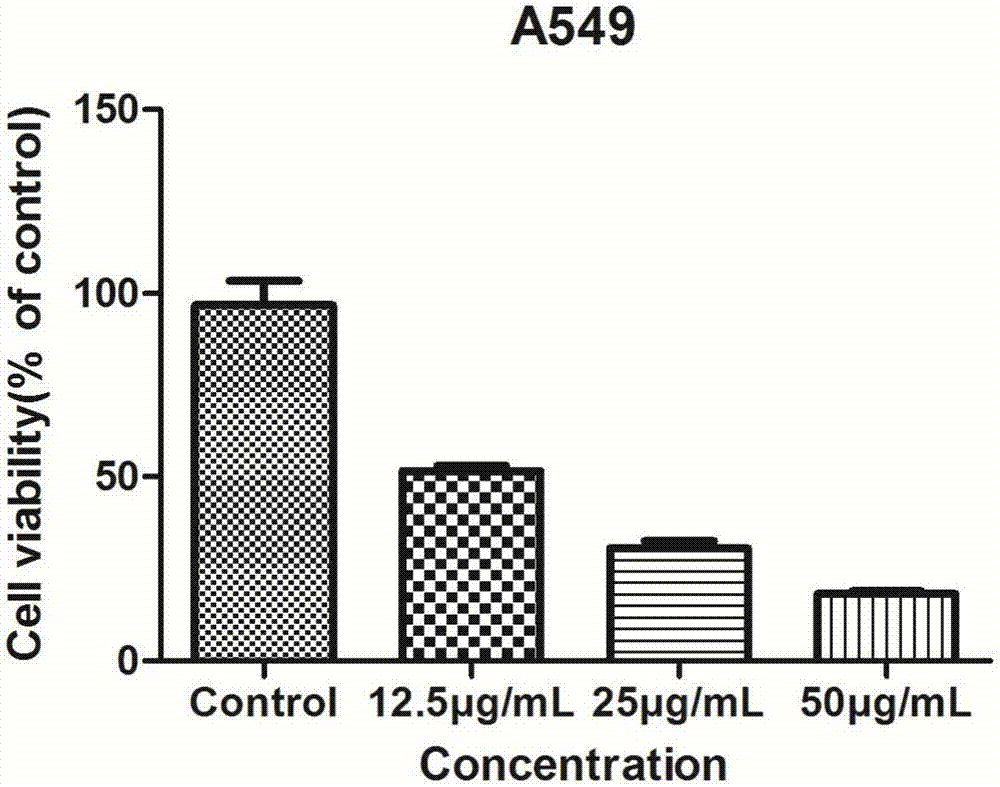
[0016] Example 1 Study on the Cell Proliferation Activity of 2-(3,4-Difluorobenzylidene) Thiosemicarbazide (I)
[0017] 1. Experimental drugs
[0018] Weigh 1mmol 3,4-difluorobenzaldehyde (3.07g) and 1.1mmol thiosemicarbazide (2.16g) and place in a 200mL reaction flask, add 20mL ethanol (95%) and stir evenly, then add 1mL glacial acetic acid dropwise, and then Reflux and stir at 65-70°C for about 10 hours, evaporate most of the ethanol under reduced pressure, add 20 ml of ice water, filter to obtain a precipitate, wash the precipitate with ice water (20 mL for three times), and recrystallize from ethanol to obtain 2-(3,4-difluorobenzene Methylene) thiosemicarbazide, white solid. 2-(3,4-Difluorobenzylidene)thiosemicarbazide is dissolved in DMSO, and prepared as a 50mg / ml storage solution for long-term storage. The maximum concentration for the experiment is 12.5, 25, and 50μg / ml.
[0019] 2. Cell lines
[0020] Human non-small cell lung cancer cells (A549) were purchased fro...
Embodiment 2
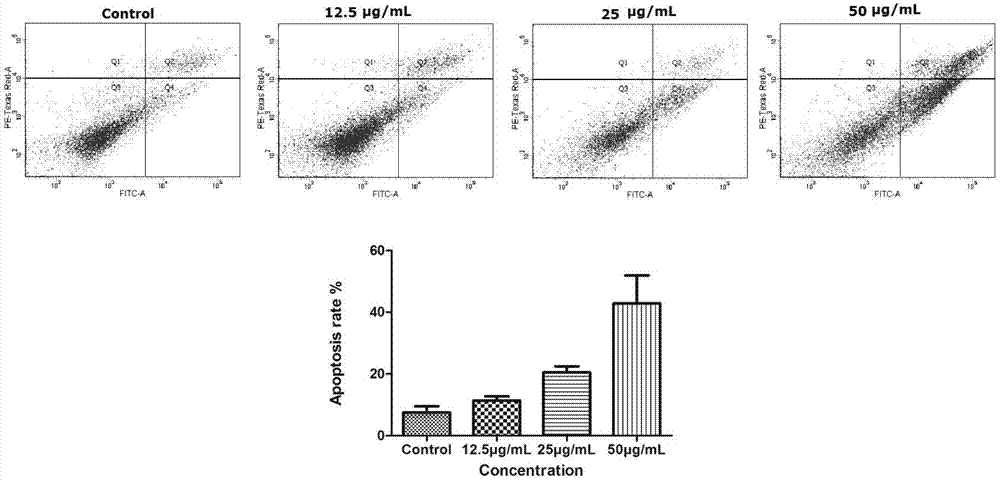
[0025] Example 2 Study on the apoptosis of A549 by 2-(3,4-difluorobenzylidene)thiosemicarbazide (I)
[0026] 1. Experimental cells:
[0027] Same as Example 1
[0028] 2. Experimental drugs:
[0029] Same as Example 1
[0030] 3. Experimental method:
[0031] Apoptosis was detected by AnnexinV / PI staining. Take A549 cells in the logarithmic phase and adjust the cell concentration to 5×10 5 / mL, seeded in a six-well plate, at 37°C, 5% CO 2 Cultivate in an incubator for 24 hours, add different concentrations of compound 2-(3,4-difluorobenzylidene)thiosemicarbazide (I) (5, 10, 20 μg / mL) for 48 hours, and set a blank group (PBS). The cells were digested with EDTA-free trypsin, washed three times with PBS, and collected 1-5×10 5 Add 500 μL of Binding Buffer to suspend the cells to form a cell suspension, then add 5 μL Annexin V-FITC and 5 μL Propidium Iodide, mix well, react at room temperature in the dark for 15 minutes, and perform detection on a flow cytometer.
[0032]...
Embodiment 3
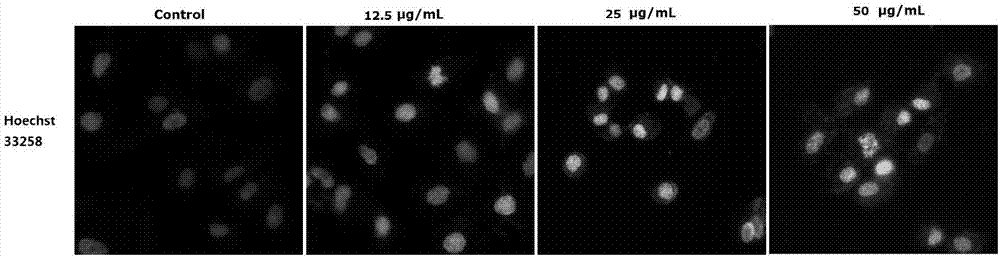
[0033] Example 3 Morphological study on apoptosis of A549 by 2-(3,4-difluorobenzylidene)thiosemicarbazide (I)
[0034] 1. Experimental cells:
[0035] Same as Example 1
[0036] 2. Experimental drugs:
[0037] Same as Example 1
[0038] 3. Experimental method:
[0039] Hoechst staining was used to detect the morphology of apoptosis. Take A549 cells in the logarithmic phase and adjust the cell concentration to 5×10 5 / mL, seeded in a six-well plate, at 37°C, 5% CO 2 After culturing in the incubator for 24 hours, add different concentrations of 2-(3,4-difluorobenzylidene)thiosemicarbazide (I) (5, 10, 20 μg / mL) for 48 hours, and set a blank group (PBS). The cells were washed three times with PBS, fixed with 4% paraformaldehyde at room temperature for 15 min, washed three times with PBS, stained with Hoechst33258 dye in the dark for 15 min, and observed under a fluorescent microscope.
[0040] The results showed that after 2-(3,4-difluorobenzylidene)thiosemicarbazide (I) a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com