Cyathin compounds, preparation method and application of cyathin compounds to neuroinflammation resisting
A technology for ovonidin and neuroinflammation, applied in the field of medicine, can solve the problems of limited curative effect, inability to restore the cognitive function of patients, and inability to prevent the pathogenesis of AD
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
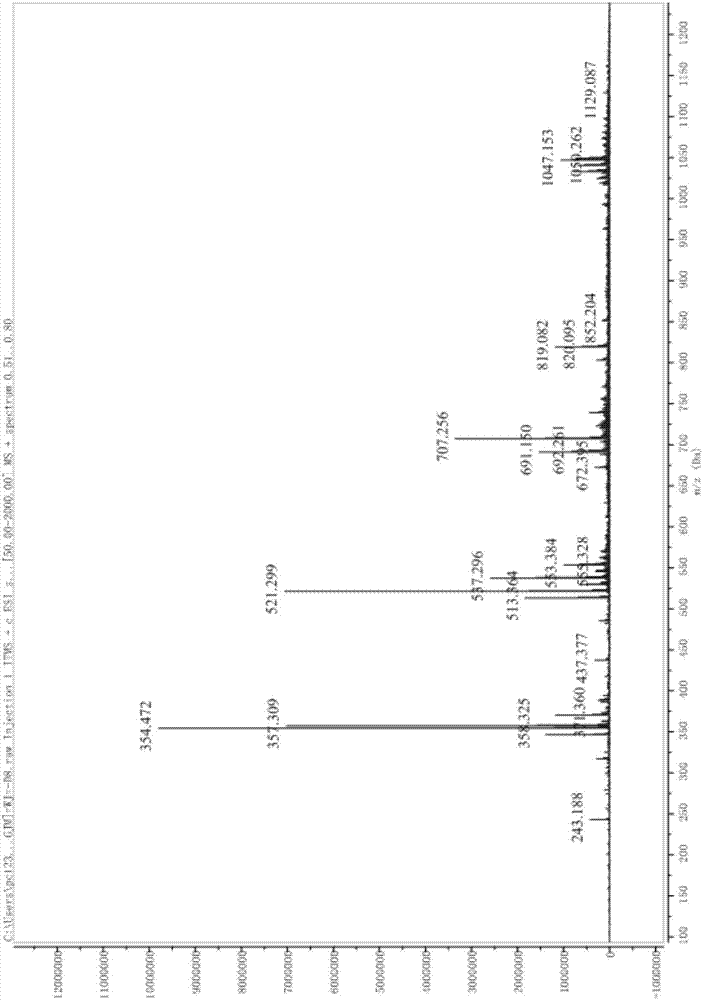
Image
Examples
Embodiment Construction
[0090] The ten compounds of the present invention, nestomycin 1-10, are isolated from the fermented liquid of Cyathus africanus. The Nestella africanum was purchased from the China General Microorganism Culture Collection and Management Center, number: CGMCC 5.1163; the strain was preserved in the Natural Medicinal Chemistry Research Center, School of Chemistry and Pharmacy, Northwest A&F University, number: (No.CA20150728).
[0091] The storage and activation culture conditions of the N. africanus are as follows: the preserved strain adopts a PDA slant medium. To activate the strain, inoculate the strain on a PDA plate, activate and cultivate it at 28°C for 7 days, until the plate is covered with mycelium. PDA plate medium: Each liter medium contains 200g potatoes, 20g glucose, and 16g agar powder.
[0092] One, the extraction method of the compound of the present invention, identification and anti-neurinflammation assay and application:
[0093] 1. Experimental materials ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com