Group of alpha-glucosidase inhibitors and application thereof
A glucosidase and inhibitor technology, applied in the field of medicinal chemistry, can solve the problems of compound separation, inability to further study or apply the market, affecting the application of Ganoderma lucidum extract, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Compound preparation:
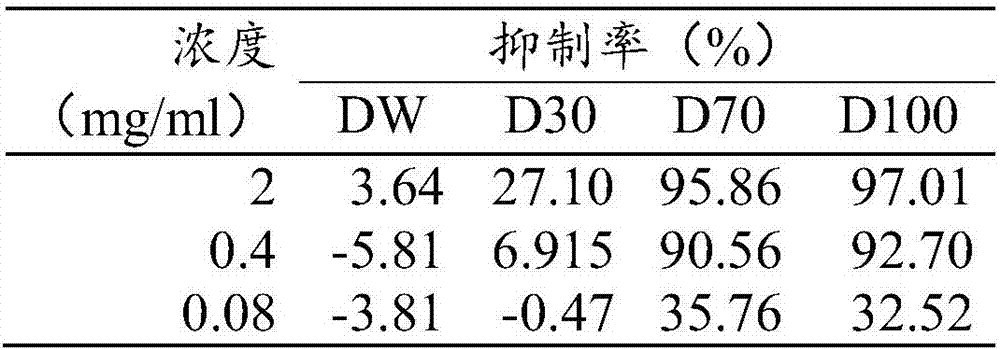
[0026] Sessile Ganoderma lucidum was powdered and extracted three times with 95% ethanol for 2 hours each time. The three extracts were combined and concentrated to obtain the extract. The Ganoderma lucidum extract was dissolved in 10% ethanol, loaded on D101 macroporous resin, and eluted with water, 30%, 70%, and 95% ethanol in sequence. The eluate was concentrated to obtain a sample eluting with water (DW), a sample eluting with 30% ethanol (D30), a sample eluting with 70% methanol (D70) and a sample eluting with 95% methanol (D100).
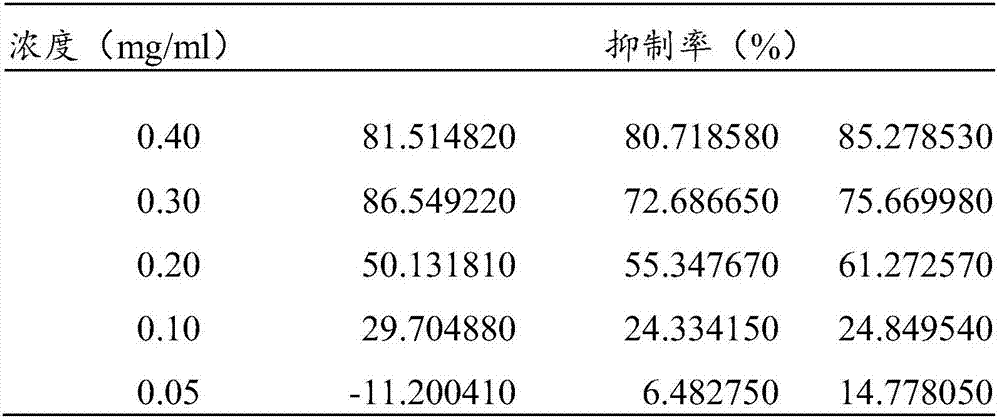
[0027]Different concentrations of Ganoderma lucidum extract were added to the microtiter plate at 20 μL per well, and triplicate wells were set up for each concentration. In addition, a drug control well, a positive reaction well and a positive control well were set up. Then add 20 μL of 0.2 U / mL α-glucosidase to drug reaction wells and positive reaction wells, and add 20 μL phosphate buffer to drug control we...
Embodiment 2
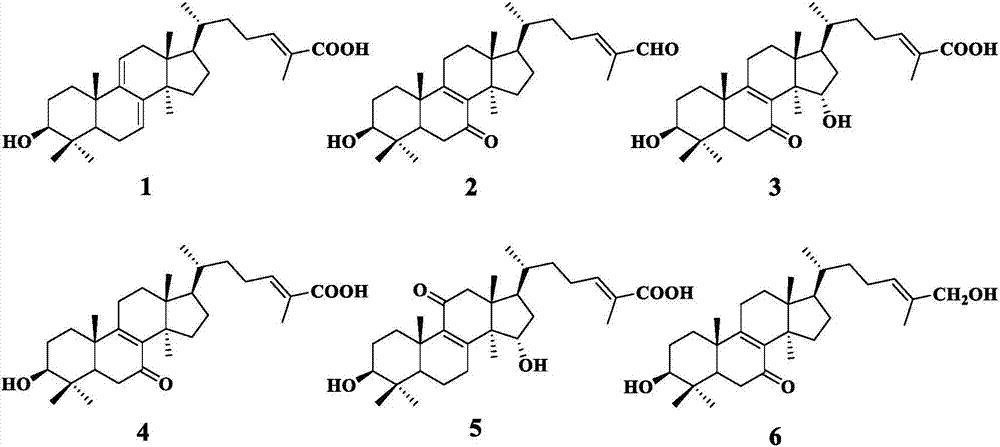
[0037] The 10% ethanol obtained by the operation of Example 1 dissolves the ganoderma lucidum extract, loads the sample on D101 macroporous resin, and successively elutes with 30% and 95% ethanol, and the 95% ethanol eluent recovers the solvent under reduced pressure to obtain the extract. The compound was chromatographed on a 500-800 mesh silica gel column, eluted with a gradient of chloroform-methanol system (100:0-0:100, v / v), and the fractions of the same components were combined to obtain 3 components (Fr1-3) ; Fr1 was subjected to silica gel column chromatography, petroleum ether-acetone (8:1-1:1, v / v) gradient elution to obtain 4 components (Fr1-1~Fr1-4); component Fr1-1 was purified by silica gel Column chromatography column, chloroform-ethyl acetate (30:1, v / v) isocratic elution to obtain compound 1. Component Fr1-2 was subjected to reverse-phase ODS column chromatography, and gradient elution of methanol-water (80:20-100:0, v / v) was used to obtain compound 2. Fr2 wa...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap