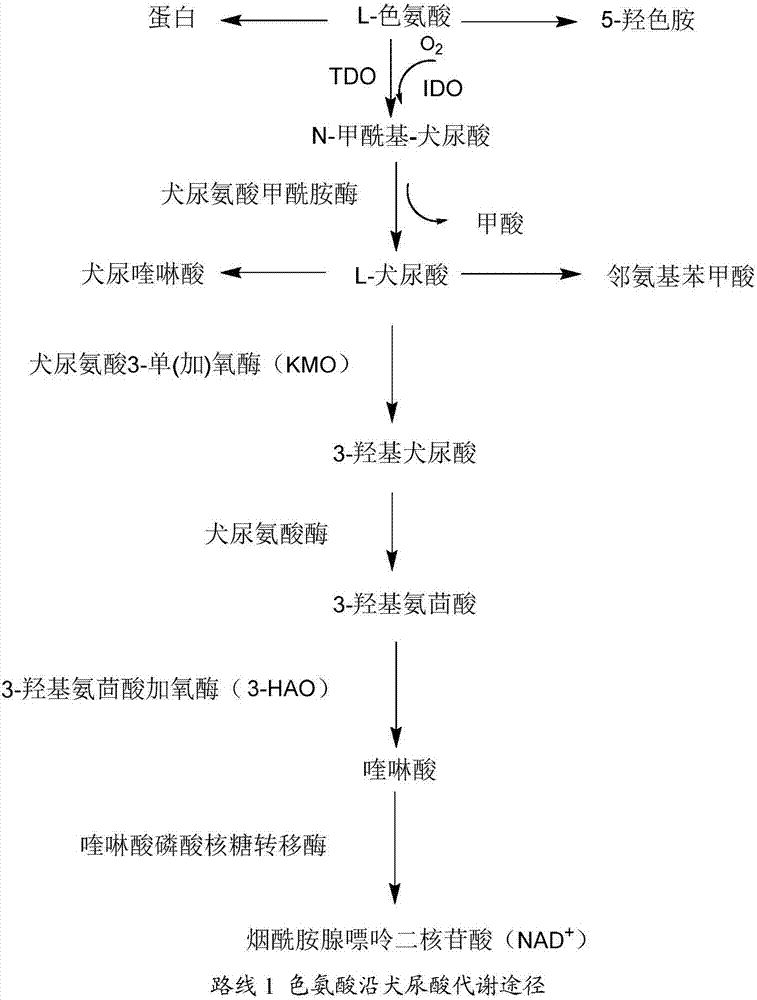
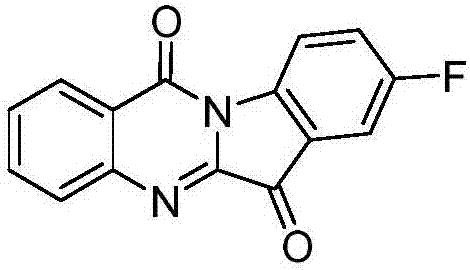
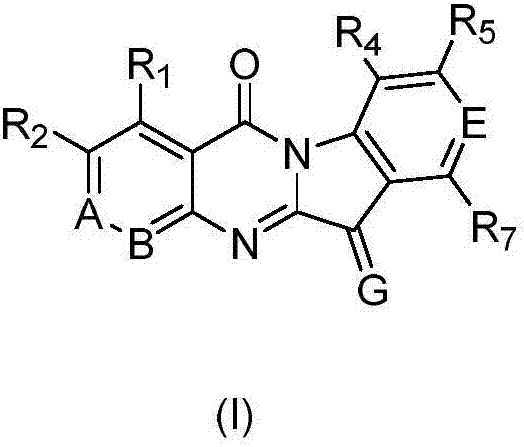
Application of azatryptanthrin derivatives as IDO1 (indoleamine 2,3-dioxygenase) and/or TDO (tryptophan 2,3-dioxygenase) inhibitors
A technology of zatryptanthin and its derivatives, which is applied in the field of use of azatryptanthin derivatives as IDO1 and/or TDO inhibitors, and can solve problems such as not being involved and showing no activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0155] Compound CY-1-1: Pyrido[2',3':4,5]pyrimido[1,2-α]indole-5,11-dione
[0156]
[0157] Isatin (0.9mmol) and DBU (2mmol) were dissolved in DMF (3ml) and stirred at room temperature for ten minutes. Dissolve 2-aminonicotinic acid (1mmol), N-methylmorpholine (1.8mmol), and HBTU (1mmol) in DMF (3ml), and add the reacted isatin and DBU solution dropwise into the solution. Stir for 20 h, monitor by TLC. After the reaction is complete, evaporate the solvent to dryness, and separate by column chromatography (dichloromethane:methanol=80:1) to obtain an orange-yellow solid with a yield of 59%. 1 H NMR (400MHz, DMSO-d 6 )δ9.08(dd, J=4.6,2.0Hz,1H),8.71(dd,J=7.9,2.0Hz,1H),8.45(d,J=8.0Hz,1H),7.90(t,J=7.9 , 4.4Hz, 2H), 7.75 (dd, J = 7.9, 4.6Hz, 1H), 7.51 (t, J = 7.6, 0.7Hz, 1H). 13 C NMR (101MHz, DMSO-d 6 )δ 182.28, 158.08, 157.20, 156.11, 147.63, 145.70, 137.90, 136.30, 127.23, 124.92, 124.70, 122.14, 119.17, 116.89.
Embodiment 2
[0159] Compound CY-1-2: 9-fluoropyrido[2',3':4,5]pyrimido[1,2-α]indole-5,11-dione
[0160]
[0161] Using 5-fluoroisatin instead of isatin, the preparation method is the same as that of CY-1-1 in Example 1. The product was a yellow solid with a yield of 51%. 1 H NMR (400MHz, DMSO-d 6 )δ9.07(s,1H),8.69(d,J=6.4Hz,1H),8.44(d,J=4.7Hz,1H),7.83(d,J=4.5Hz,1H),7.75(d, J=7.5Hz, 2H). 13 C NMR (101MHz, DMSO-d 6 )δ 181.85, 160.13, 158.53, 157.46, 156.72, 142.41, 136.83, 125.34, 124.62, 124.34, 119.54, 119.23, 112.48, 112.18.
Embodiment 3
[0163] Compound CY-1-3: 9-(trifluoromethoxy)pyrido[2',3':4,5]pyrimido[1,2-α]indole-5,11-dione
[0164]
[0165] Using 5-trifluoromethoxy isatin instead of isatin, the preparation method is the same as that of CY-1-1 in Example 1. The product was a light yellow solid with a yield of 29%. 1 H NMR (400MHz, DMSO-d 6 )δ9.09(d, J=3.7Hz, 1H), 8.71(d, J=7.7Hz, 1H), 8.54(d, J=8.7Hz, 1H), 7.97(s, 1H), 7.92(d, J=8.7Hz, 1H), 7.77 (dd, J=7.7, 4.6Hz, 1H). 13 C NMR (101MHz, DMSO-d 6 )δ 181.56, 158.48, 157.55, 156.84, 148.22, 146.96, 144.61, 136.89, 130.69, 125.40, 124.39, 121.75, 119.46, 119.19, 119.06, 118.38.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com