Short-chain peptide derived from tumor antigen PRAME
A complex, amino acid technology, used in tumor-specific antigens, tumor rejection antigen precursors, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
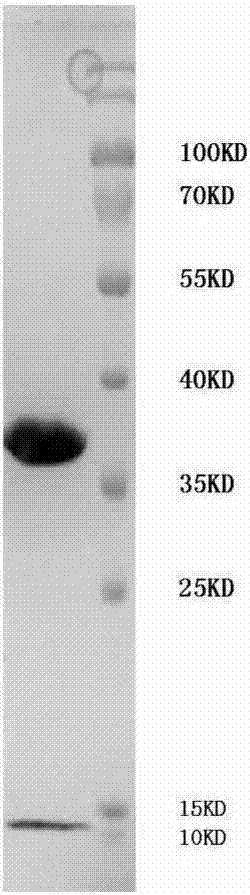
[0084] The preparation methods of the above TCR and antibody are known to those skilled in the art, including but not limited to, expressing and purifying from Escherichia coli cells or insect cells.
[0085] In another aspect, the present invention further provides the use of the peptides, pMHC complexes, nucleic acid molecules, vectors, cells and binding molecules of the present invention in pharmaceuticals. The peptide, pMHC complex, nucleic acid, vector, cell or binding molecule can be used to treat or prevent cancer, preferably melanoma, bladder cancer, liver cancer, epidermoid cancer, non-small cell lung cancer and squamous cell carcinoma, etc.
[0086] The present invention also provides a pharmaceutical composition, which comprises the peptide of the present invention, the pMHC complex, the nucleic acid molecule of the present invention, the cell of the present invention or the binding molecule of the present invention, and a pharmaceutically acceptable carrier. The ph...
Embodiment 1
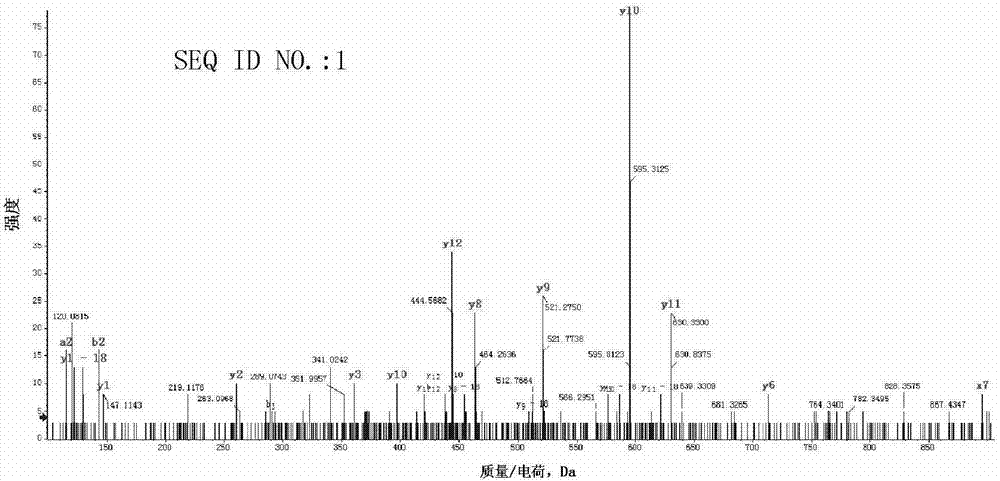
[0093] Example 1 Identification of polypeptides derived from PRAME antigens by mass spectrometry
[0094] Before the identification by mass spectrometry, the present invention further verifies that the PRAME antigen is expressed in various tumor cells. Specifically, a digital single-molecule multiplex gene expression profiling system is used for detection (nanostring). The results showed that PRAME antigen was expressed in tumor cells such as melanoma, adenocarcinoma, non-small cell lung cancer, hepatocellular carcinoma, myeloma, osteosarcoma, renal cell carcinoma, and lymphoma.
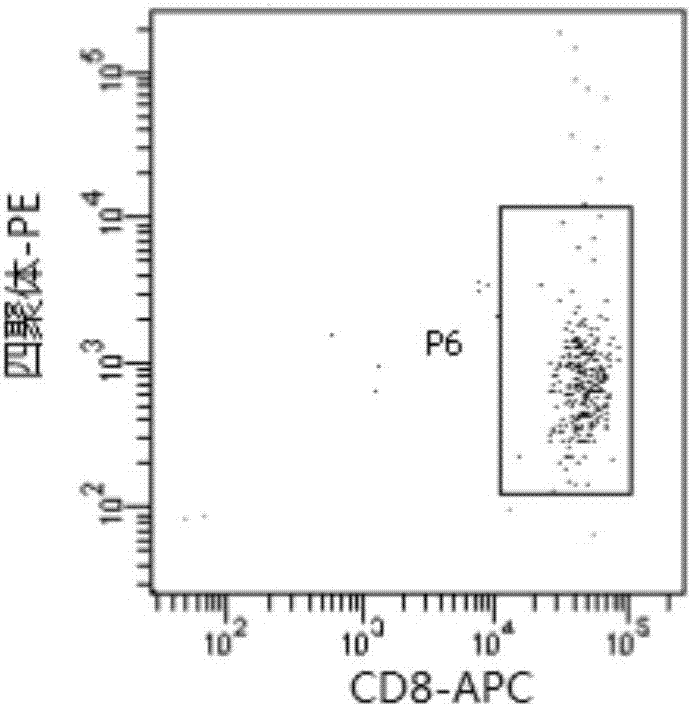
[0095]The HLA-short peptide complex was purified using commercial antibody W6 / 32. Specifically, the tumor cells were lysed with a buffer solution containing the non-ionic surfactant Triton X-100 (1% v / v), 1 ml of the lysate was added to 2*10^7 cells, and the tumor cells were incubated at 4° C. for 1 hour by rolling. The cell debris was removed by centrifugation, and the supernatant was first incuba...
Embodiment 2
[0102] The preparation of embodiment 2 soluble pMHC complexes
[0103] The heavy chain and light chain (β2m) of type I HLA-A*1101 molecules were expressed in E. coli in the form of inclusion bodies. It should be noted that the heavy chain of the HLA-A*1101 molecule used in this example does not contain its transmembrane and cytoplasmic regions in order to obtain soluble pMHC complexes. In addition, in order to facilitate the subsequent biotinylation of the soluble pMHC complex, a biotinylation tag can be added to the C-terminus of the heavy chain. The specific process of preparing the soluble pMHC complex of the present invention is as follows:
[0104] a.Purification
[0105] Collect 100ml of E.coli bacteria liquid induced to express heavy chain or light chain, centrifuge at 8000g at 4°C for 10min, wash the bacteria once with 10ml PBS, then resuspend the bacteria with 5ml BugBuster Master Mix Extraction Reagents (Merck) vigorously shake, and place in Rotate and incubate at...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com