Fischer-Tropsch synthesis iron-based catalyst and preparation method thereof
An iron-based catalyst, Fischer-Tropsch synthesis technology, applied in chemical instruments and methods, physical/chemical process catalysts, chemical elements of heterogeneous catalysts, etc. High Fischer-Tropsch reactivity and other problems, to achieve the effects of excellent selectivity, reduced methane selectivity, and easy control of components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
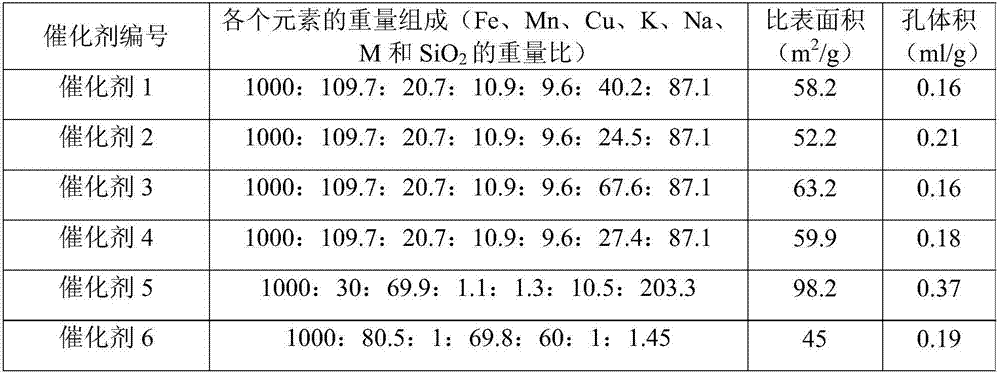
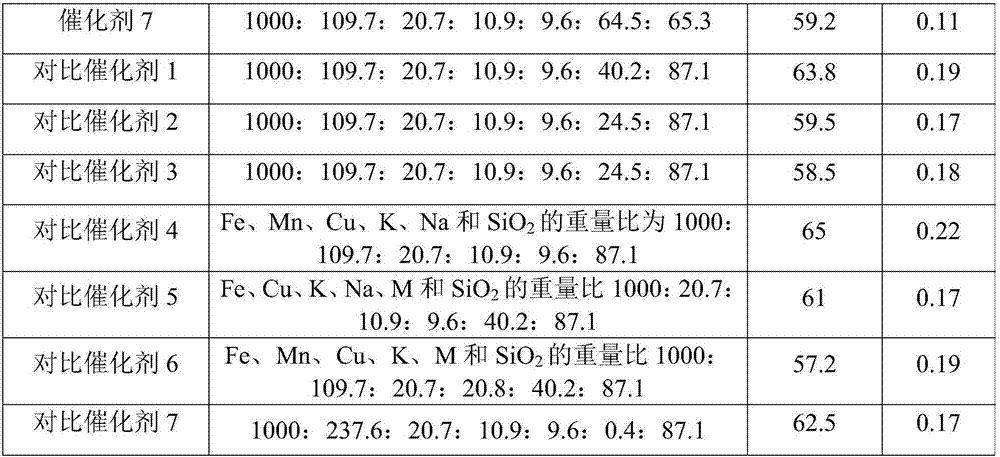
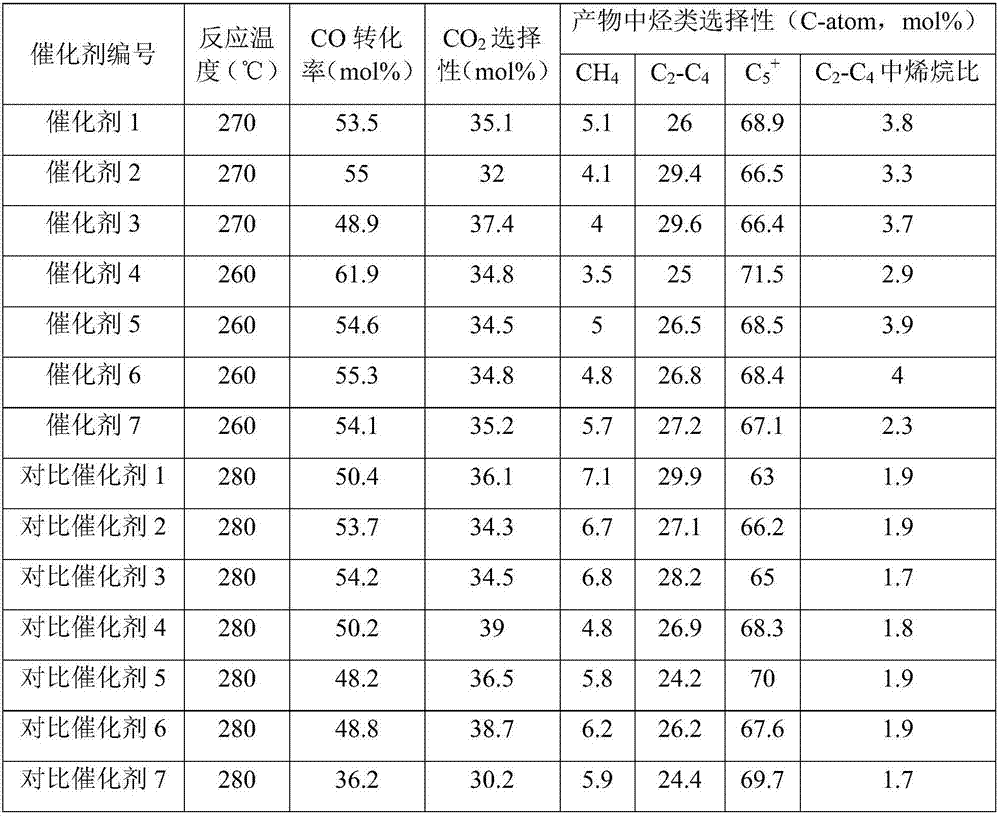
[0058] Example 1
[0059] (1) Preparation of silica sol pretreated by the precursor of the carrier modifier Ca
[0060] Add 10g of Ca(NO 3 ) 2 ·4H 2 O is dissolved in 50g deionized water, fully dissolved; take SiO 2 12g of alkaline silica sol with a content of 30.5% by weight; at room temperature, the dissolved Ca(NO 3 ) 2 The solution was slowly added dropwise to the continuously vigorously stirred silica sol, and the solution was added within 10 minutes; after continuous stirring and mixing for 30 minutes, ammonia water with a concentration of 5% by weight was slowly added dropwise to the vigorously stirred silica sol until the pH of the system The value is 11, and stirring is continued for 30 minutes to obtain a silica sol pretreated with the precursor of the carrier modifier Ca.
[0061] (2) Preparation of Fe-Tropsch synthesis iron-based catalyst
[0062] Fe(NO 3 ) 3 ·9H 2 O solid 303g and 30g 50wt% Mn(NO 3 ) 2 Add the solution to 750ml deionized water to fully dissolve; place the...
Example Embodiment
[0063] Example 2
[0064] (1) Preparation of silica sol pretreated by the precursor of the carrier modifier Mg
[0065] Add 11g of Mg(NO 3 ) 2 ·6H 2 O is dissolved in 50g deionized water, fully dissolved; take SiO 2 12g of alkaline silica sol with a content of 30.5% by weight; at room temperature, the dissolved Mg(NO 3 ) 2 The solution was slowly added dropwise to the continuously vigorously stirred silica sol, and the solution was added within 10 minutes; after continuous stirring and mixing for 30 minutes, ammonia water with a concentration of 5% by weight was slowly added dropwise to the vigorously stirred silica sol until the pH of the system The value is 11, and stirring is continued for 30 minutes to obtain a silica sol pretreated with a precursor of the carrier modifier Mg.
[0066] (2) Preparation of Fe-Tropsch synthesis iron-based catalyst
[0067] The process of step (2) of Example 1 was repeated to prepare a catalyst, and the obtained catalyst was named Catalyst 2.
Example Embodiment
[0068] Example 3
[0069] (1) Preparation of silica sol pretreated by the precursor of the carrier modifier Zn
[0070] 13g of Zn(NO 3 ) 2 ·6H 2 O is dissolved in 50g deionized water, fully dissolved; take SiO 2 12g alkaline silica sol with a content of 30.5% by weight; at room temperature, the dissolved Zn(NO 3 ) 2 The solution was slowly added dropwise to the continuously vigorously stirred silica sol, and the dripping of the solution was completed within 10 minutes; after continuous stirring and mixing for 30 minutes, 5 wt% ammonia water was slowly added dropwise to the vigorously stirred silica sol until the pH value of the system The value is 11, and the stirring is continued for 30 minutes to obtain a silica sol pretreated with a precursor of the carrier modifier Zn.
[0071] (2) Preparation of Fe-Tropsch synthesis iron-based catalyst
[0072] The process of step (2) of Example 1 was repeated to prepare a catalyst, and the obtained catalyst was named Catalyst 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com