Reactive emulsifier, preparation method and application thereof
A technology of reactive emulsifiers and compounds, applied in the direction of coating, etc., can solve the problems of reduced product reactivity, high reaction temperature, and reduced effective double bond content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
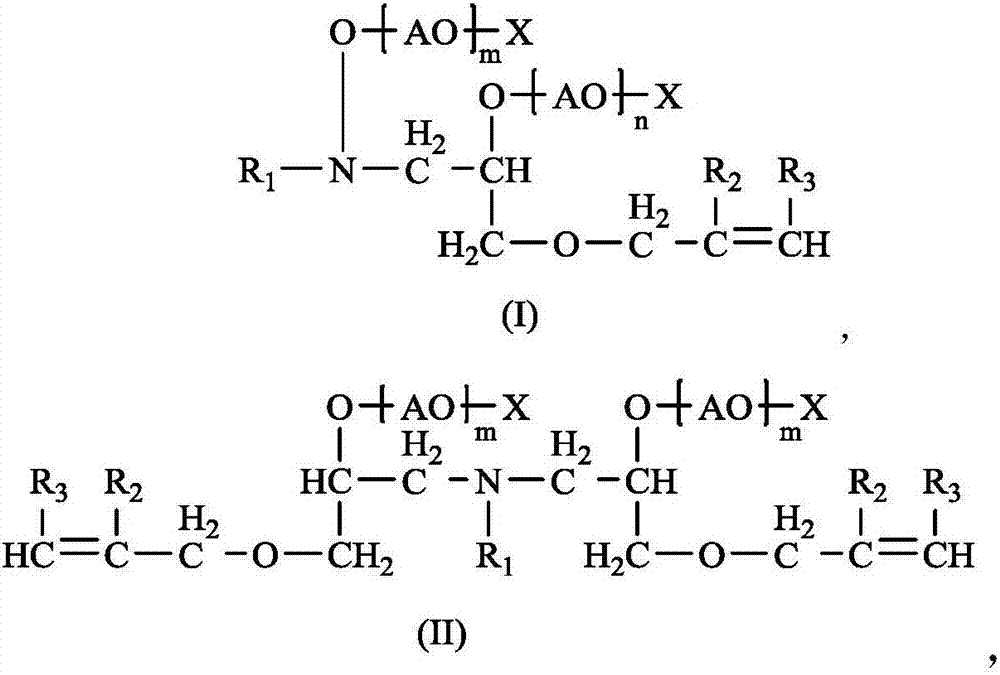
Image
Examples
Embodiment 1
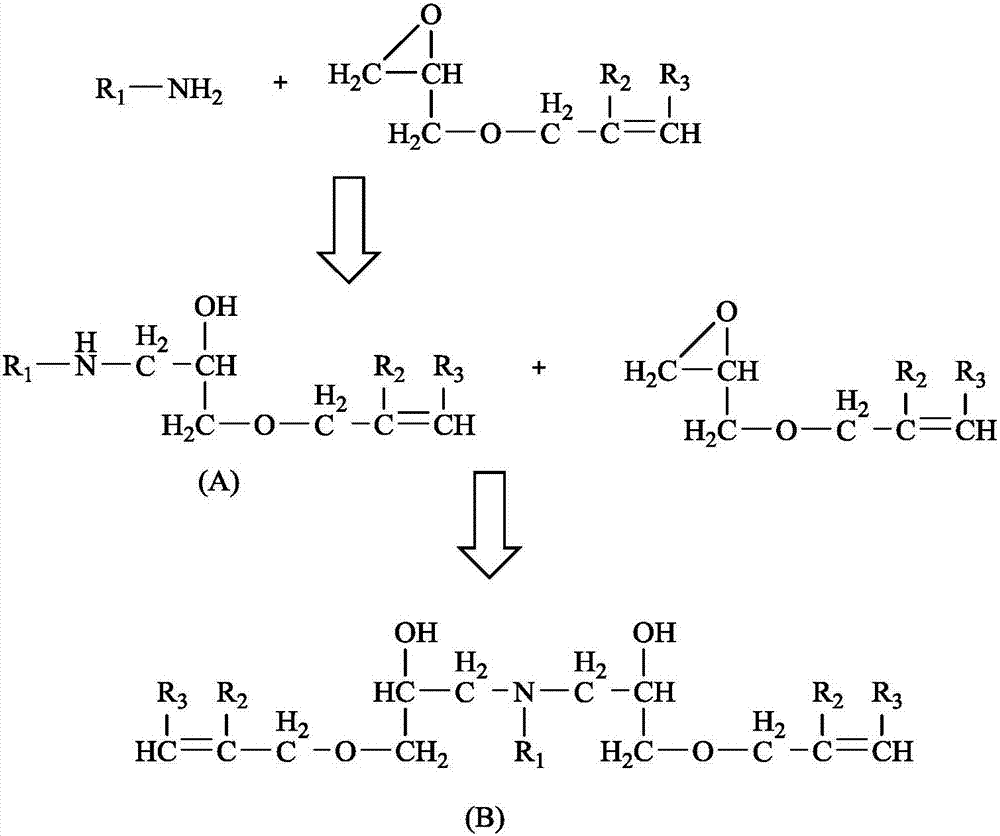
[0047] Using coconut oil primary amine (C12 / 14 primary amine, C) react with allyl glycidyl ether, the reaction pressure is normal pressure, the molar ratio of coconut oil primary amine and allyl glycidyl ether is 1 / 1.05, and the molar weight of coconut oil primary amine can be according to The amine value index of C product (272~285mgKOH / g) or the actual measured amine value calculation.
[0048] Weigh 200g of water up to standard ( C Coconut oil primary amine (measured total amine value 275mgKOH / g) is put into the reaction kettle, and under the protection of nitrogen, stir and heat up to 30°C. Slowly add a total of 117.56 g of allyl glycidyl ether to the reaction kettle dropwise, the dropwise addition is completed within 1 hour, continue to stir for 7 hours, lower the temperature, and take a sample of 1-1#.
Embodiment 2
[0050] With reference to the method of Example 1, the molar ratio of coconut oil primary amine and allyl glycidyl ether is 1 / 1.05. Weigh the coconut oil primary amine into the reaction kettle, stir and raise the temperature to 90°C under the protection of nitrogen. Slowly add allyl glycidyl ether dropwise to the reaction kettle, the dropwise addition is completed within 1 hour, continue to stir for 3 hours, lower the temperature, and take samples as 1-2# samples.
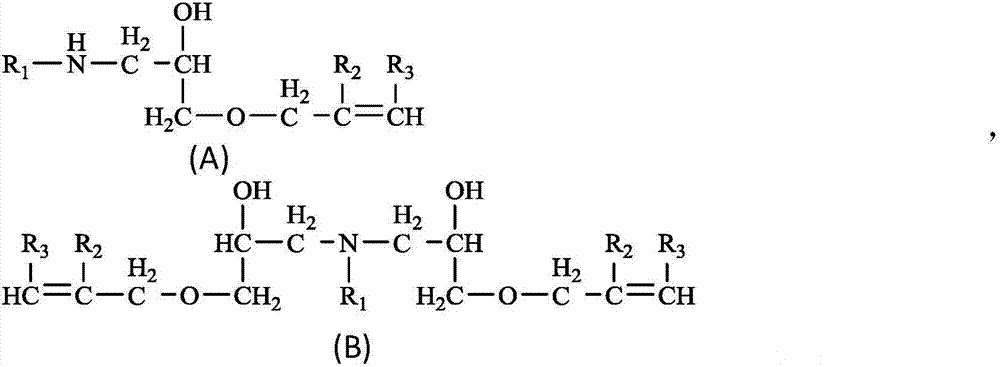
[0051] According to the feeding ratio of coconut oil primary amine and allyl glycidyl ether in embodiment 1 and embodiment 2, gained 1-1# sample and 1-2# sample are mainly made up of the compound of following formula (A-1) structure,
[0052]
[0053] where R 1 It is mainly an alkyl group with 8 to 16 carbon atoms, because the fatty primary amine raw material comes from natural coconut oil.
Embodiment 3
[0055] With reference to the method of Example 1, the molar ratio of coconut oil primary amine and allyl glycidyl ether is 1 / 2.0. Weigh the coconut oil primary amine into the reaction kettle, stir and raise the temperature to 30°C under the protection of nitrogen. Slowly add allyl glycidyl ether dropwise to the reaction kettle, the dropwise addition is completed within 2 hours, continue to stir for 6 hours, lower the temperature, and take samples as 1-3# samples.
[0056] According to the feeding ratio of coconut oil primary amine and allyl glycidyl ether in embodiment 3, gained 1-3# sample mainly is made up of formula (B-1) structure compound,
[0057]
[0058] where R 1 It is mainly an alkyl group with 8 to 16 carbon atoms.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com