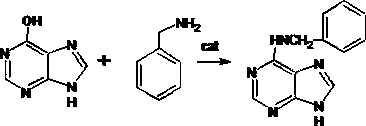A kind of preparation method of 6-benzylaminopurine
A technology of benzylaminopurine and benzylamine, which is applied in the field of preparation of 6-benzylaminopurine, can solve the problems of product purity reduction and difficult separation, and achieve the effects of high product purity, simplified reaction, and short reaction route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Add 0.1mol (13.6g) hypoxanthine, 0.18mol (19.2g) benzylamine, 5g (I 2 -P 2 o 5 -ZnCl 2 ) catalyst and DMF solvent 200mL, make P 2 o 5 : ZnCl 2 :I 2 The mass ratio is 1:0.3:0.01. Then, heat up to about 70°C, and control the temperature at 70°C for 5 hours. After the reaction, slowly add 1mol (18g) of process water and a small amount of water , is more conducive to the removal of salt components, especially to reduce the influence of chloride ions in the catalyst, and control the temperature at about 70°C to adjust the pH value to neutral. The crude product of 6-benzylaminopurine (6-BA) was 21.9g after drying, the converted yield was 97.22%, and the content was 98.2%. The obtained crude product is tested for chloride ion content, and the content of chloride ion is ≤0.1 ppm. In order to better improve the purity of the product, further refining treatment can be carried out, specifically: add 20 g of the crude product of 6-benzylaminopurine (6-BA) obtained above and...
Embodiment 2
[0023] Add 0.1mol (13.6g) hypoxanthine, 0.15mol (16g) benzylamine, 4g (I 2 -P 2 o 5 -ZnCl 2 ) catalyst and DMF solvent 100mL, make P 2 o 5 : ZnCl 2 :I 2The mass ratio is 1:0.2:0.02. Then, heat up to about 75°C, and control the temperature at 75°C for 4 hours. After the reaction, slowly add 1mol (18g) of process water dropwise, and control the temperature at 75°C Adjust the pH value to neutral at about 75°C. After the adjustment, a large amount of solids are precipitated, and they are directly subjected to suction filtration while hot to obtain the crude product of 6-benzylaminopurine (6-BA). After drying, it is 21.7g, equivalent to yield The rate is 96.2%, and the content is 98.1%. The obtained crude product is tested for chloride ion content, and the content of chloride ion is ≤0.1 ppm. In order to better improve the purity of the product, further refining treatment can be carried out, specifically: add 20 g of the crude product of 6-benzylaminopurine (6-BA) obtained ...
Embodiment 3
[0025] Add 0.1mol (13.6g) hypoxanthine, 0.18mol (21.4g) benzylamine, 6.8g (I 2 -P 2 o 5 -ZnCl 2 ) catalyst and DMF solvent 100mL, make P 2 o 5 : ZnCl 2 : I 2 The mass ratio is 1:0.25:0.01. Then, heat up to about 50°C, and control the temperature at 50°C for 6 hours. After the reaction, slowly add 1mol (18g) of process water dropwise, and control the temperature at 50°C Adjust the pH value to neutral at about 55°C. After the adjustment, a large amount of solids are precipitated, and they are directly subjected to suction filtration while hot to obtain the crude product of 6-benzylaminopurine (6-BA). After drying, it is 20.8g, equivalent to yield The rate is 96.8%, and the content is 98.3%. The obtained crude product is tested for chloride ion content, and the content of chloride ion is ≤0.1ppm. In order to better improve the purity of the product, further refining treatment can be carried out, specifically: add 20 g of the above-mentioned crude product of 6-benzylaminop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com