Cruciate ligament regenerative implant and its preparation method and application
A cruciate ligament, regenerative technology, applied in ligaments, medical science, prosthesis, etc., can solve problems such as long operation time, limited supply of grafts, and limited sources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Combination of mechanical support structures and determination of maximum tensile force
[0064] Construct mechanical support structures 1-4 as follows:
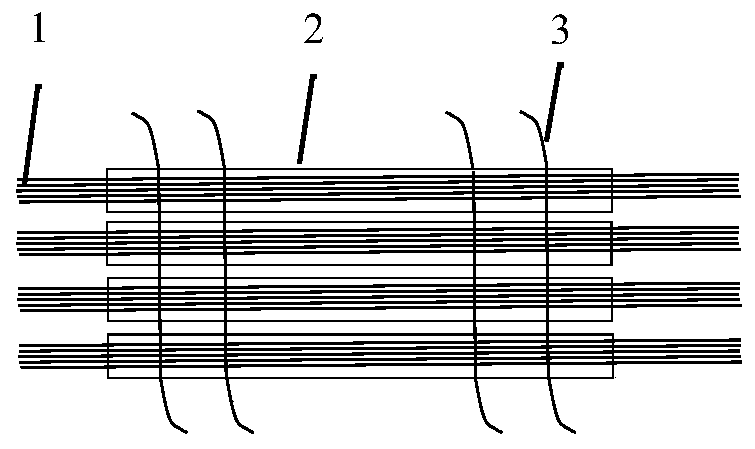
[0065] Take 90 sutures of 500mm long USP7-0PDSII (diameter: 0.07mm) and weave them to form a diameter of 0.66mm and a cross-sectional area of 0.35mm 2 The mechanical support structure 1;
[0066] Take 80mm long USP7-0 PDSII (diameter: 0.07mm) sutures 10, after weaving, the diameter is 0.22mm, and the cross-sectional area is 0.04mm 2 Mechanical support structure 2;
[0067] Take 1 USP2-0PDSII suture with a length of 100mm, and form a diameter (mean value) of 0.34mm and a cross-sectional area of 0.09mm 2 Mechanical support structure 3;
[0068] Take a 350mm long USP2-0PDSII (diameter: 0.34) to form a diameter of 0.34mm and a cross-sectional area of 0.09mm 2 Mechanical support structure4.
[0069]Fix 6 pieces of each of the above mechanical support structures 1-4 on a mechanical analyzer (Instron 5...
Embodiment 2
[0073] Example 2 Combination of Regeneron and Measurement Results of Tensile Strength
[0074] Construct Regeneron 1-4 as follows:
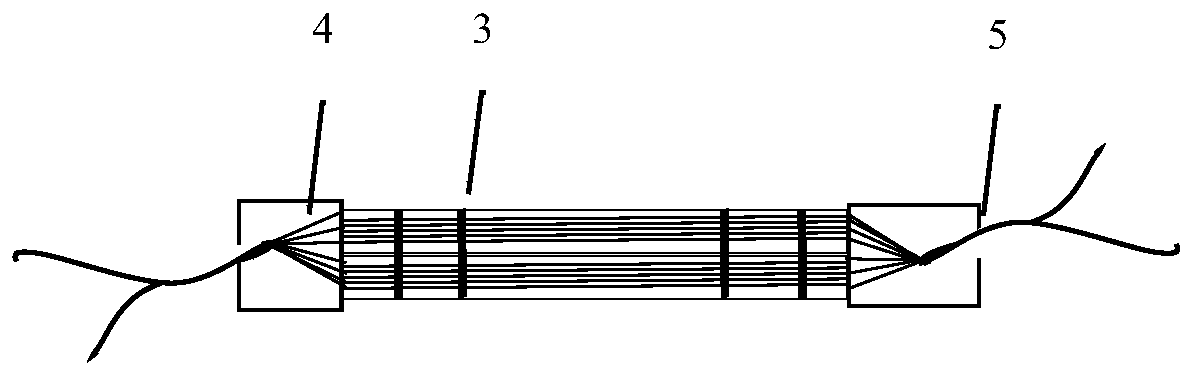
[0075] Regeneron-1: Take 90 sutures of 50mm long USP7-0PDSII (diameter: 0.07mm), after weaving, the diameter is 0.66mm, and the cross-sectional area is 0.35mm 2 The mechanical support structure; take the electrospun support material (length: 100mm) with a thickness of 0.2-0.25mm to wrap the mechanical support structure layer by layer, so that the final diameter is 1.00mm, of which: the cross-sectional area of the regenerated element is 0.79mm 2 , the regenerative cross-sectional area is 0.44mm 2 , the regeneration ratio is: 56%;
[0076] Regeneron-2: Take 10 80mm long USP7-0PDSII (diameter: 0.07mm) sutures to form a diameter of 0.22mm and a cross-sectional area of 0.04mm 2 The mechanical support structure; take the electrospun support material (length: 100mm) with a thickness of 0.2-0.25mm to wrap the mechanical support structure layer by ...
Embodiment 3
[0088] Example 3 Combination of Regeneron Sets and Measurement of Mechanical Strength
[0089] Regeneron sets 1-4 (i.e. cruciate ligament regenerative implants) were constructed as follows:
[0090] Regeneron set-1: Take a total of 20 regeneron-1 prepared in Example 2, and combine them into bundles to form a diameter of 4mm, and the cross-sectional area of the regeneron set is 16mm 2 The regenerator set-1;
[0091] Regeneron Set-2: Take a total of 25 Regeneron-2 prepared in Example 2, and combine them into bundles to form a diameter of 10mm, and the cross-sectional area of the Regeneron Set is 79mm 2 Regeneron set-2;
[0092] Regeneron set-3: Take 20 pieces of Regeneron-3 prepared in Example 2, combine them into bundles, form a diameter of 9mm, and the cross-sectional area of the regeneron set is 60mm 2 Regeneron set-3;
[0093] Regeneron set-4: Take a total of 62 regeneron-4 prepared in Example 2, combine them into bundles, form a diameter of 5mm, and regeneron set ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com