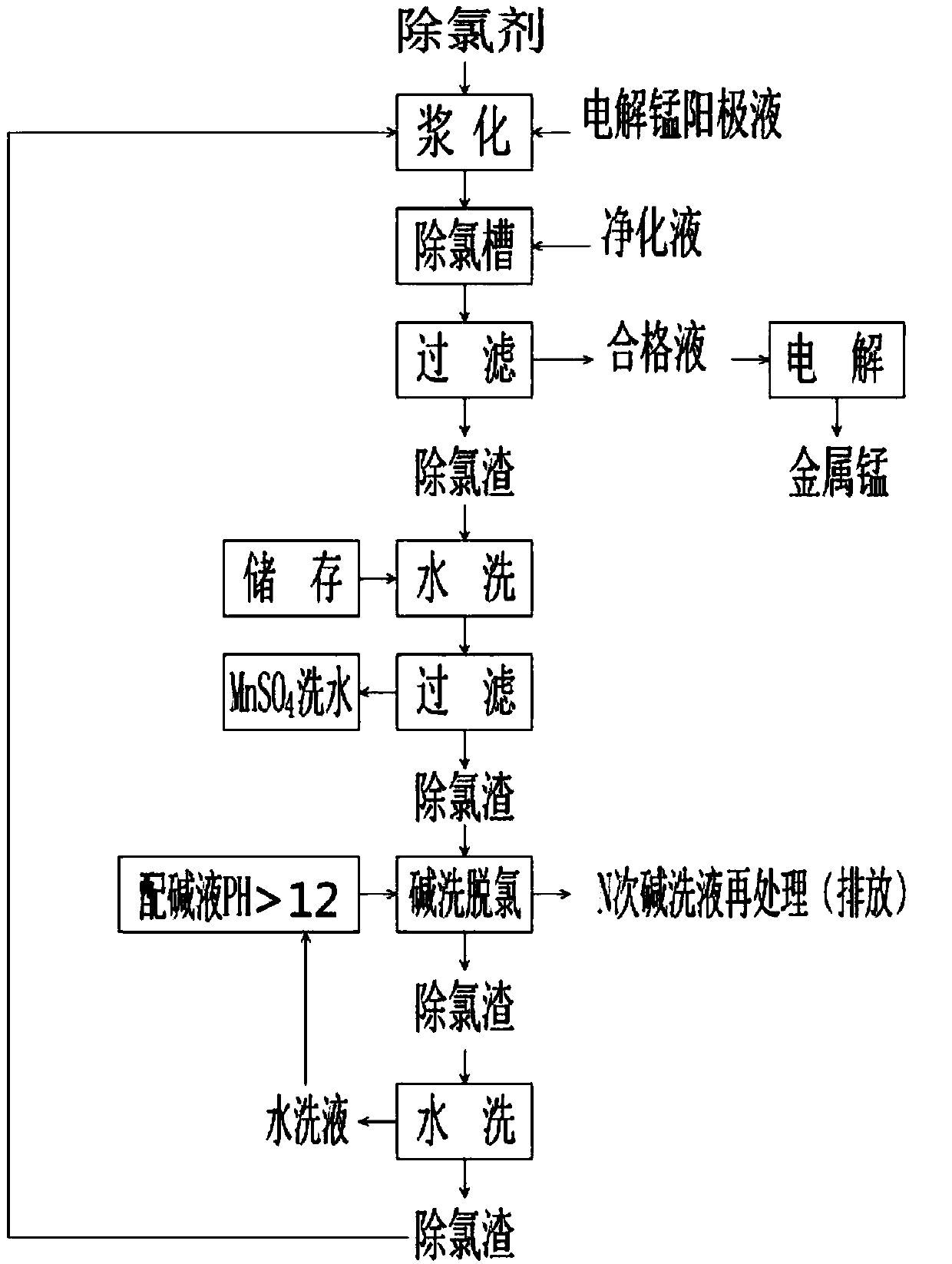
Method for removing chloride ions in electrolytic manganese solution
A technology for electrolysis of manganese and chloride ions, applied in the field of manganese hydrometallurgy, can solve problems such as large loss of manganese resources, closure of production enterprises, increase in production costs, etc., and achieve good regeneration capacity, low loss, and small amount of chlorine removal residue. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1, 17 grams of R-Bi 2 O 3 Type chlorine removal agent is added to 68g of electrolytic manganese anode solution with 35g / l sulfuric acid content, start the stirrer, stir for 20 minutes to slurry, adjust the PH value of the slurry reaction end point with dilute sulfuric acid to 2, and add 1000 ml of slurry In the electrolytic manganese solution with chloride ion content of 1160 mg / L to be treated, fully stir for 60 minutes at 52°C at a stirring rate of 60 revolutions / minute, adjust the pH value of the chlorine removal reaction end point to 4.6 with dilute sulfuric acid, and filter ; The chloride ion content in the filtrate is 87 g / L, and the chlorine removal rate is 92.5%.
Embodiment 2
[0033] Example 2, 18 grams of R-Bi 2 O 3 Type dechlorination agent is added to 90g sulfuric acid content of 40g / l electrolytic manganese anode solution, start the stirrer, stir for 25 minutes to slurry, adjust the PH value of the slurry reaction end point with dilute sulfuric acid to 3, and add 1000 ml of slurry In the electrolytic manganese solution with a chloride ion content of 1230 mg / L to be treated, fully stir for 45 minutes at 55°C at a stirring rate of 60 revolutions per minute, adjust the pH value of the end point of the dechlorination reaction with dilute sulfuric acid to 4.8, and filter ; The chloride ion content in the filtrate is 71.34 g / L, and the chlorine removal rate is 94.2%.
Embodiment 3
[0034] Example 3, 11 grams of R-Bi 2 O 3 Type dechlorination agent is added to 66g of electrolytic manganese anode solution with sulfuric acid content of 30g / l, start the stirrer, stir for 30 minutes to slurry, adjust the PH value of the slurry reaction end point with dilute sulfuric acid to 1, and add 1000 ml of slurry In the electrolytic manganese solution with a chloride ion content of 863 g / L to be treated, fully stir for 55 minutes at 51°C at a stirring rate of 60 rpm, and adjust the pH value of the end point of the dechlorination reaction to 4.4 with dilute sulfuric acid. The filtrate The chloride ion content is 100.11 g / L, and the chlorine removal rate is 88.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com