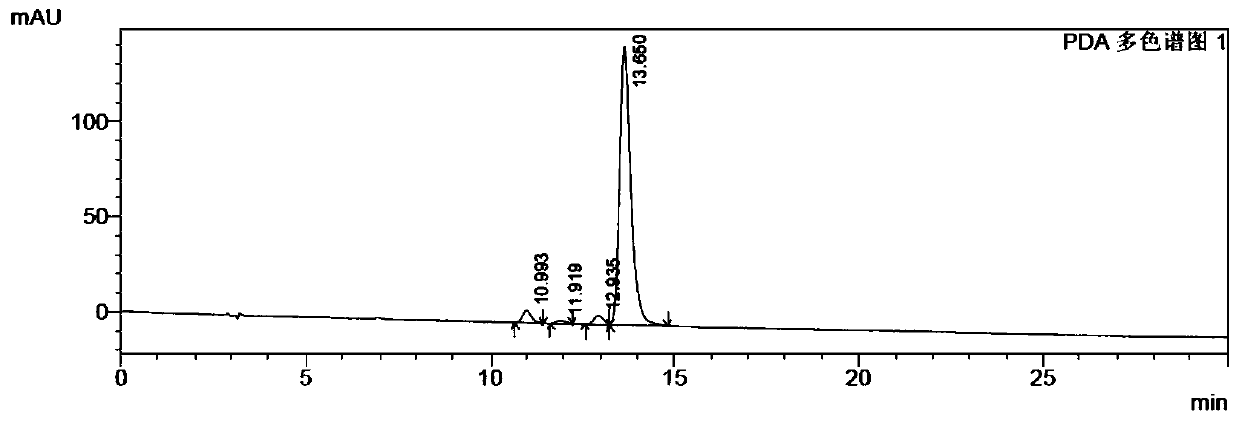
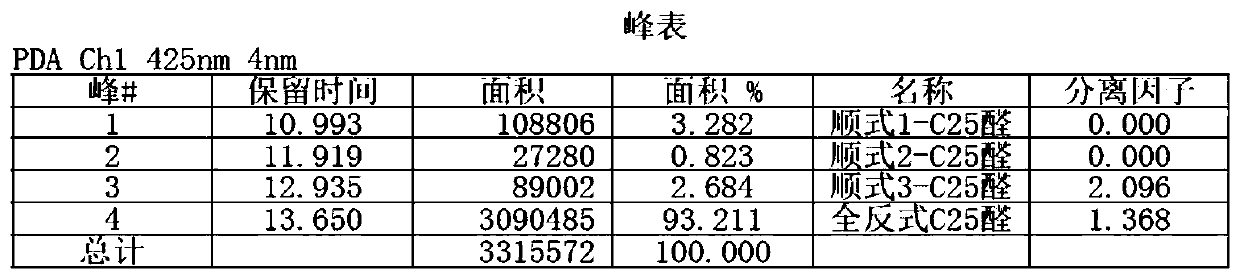
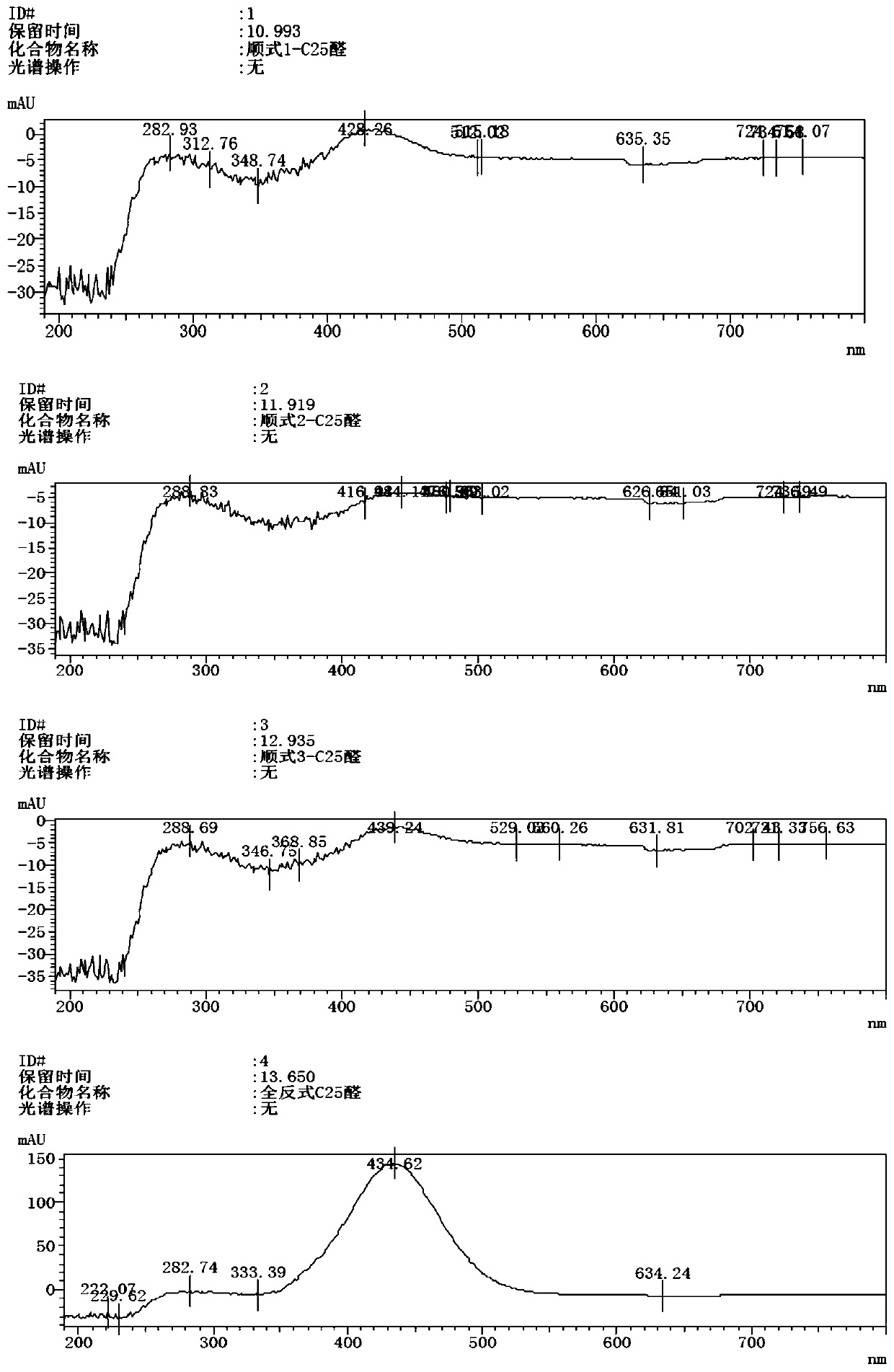
A kind of preparation method of β-Apo-12'-carotaldehyde
A carrot aldehyde, dripping technology, applied in the direction of organic chemistry, can solve the problems of high cost, expensive, unsuitable for industrial production, etc., and achieve the effect of low cost, less manpower, material and financial resources, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A preparation method of β-Apo-12'-carotaldehyde, comprising,
[0032] Step 1): Under the protection of an inert gas, sodium hydride and dimethyl sulfoxide react to obtain dimethyl sulfoxide sodium salt;
[0033] Step 2): Under the protection of inert gas, 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienyl phosphoric acid di Ethyl ester (pentadecyl phosphate) is dissolved in an ether solvent or a dipolar aprotic solvent, and then the dimethyl sulfoxide sodium salt obtained in step 1) is added dropwise therein for transposition, and the reaction formula is as in formula I:
[0034]
[0035] Step 3): Dissolve 2,7-dimethyl-2,4,6-octatriene-1,8-dialdehyde (C10-dialdehyde) in ether solvent or dipolar aprotic solvent, and then Add the transpositioned 3-methyl-5-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2,4-pentadienyl phosphoric acid obtained in step 2) dropwise Diethyl ester, the two undergo Wittig-Horner condensation reaction to obtain β-apo-12'-carotaldehyde, the...
Embodiment 1
[0051] A preparation method of β-Apo-12'-carotaldehyde, comprising the following steps:
[0052] step 1):
[0053] Under the protection of nitrogen, add 0.15 mol of sodium hydride solid into a 500mL reaction bottle, and slowly add 0.5 mol of dimethyl sulfoxide dropwise under magnetic mechanical stirring. After about 30 minutes, the dropwise addition is completed, and the internal temperature rises to 60-65°C for reaction. , the bubbles in the system gradually increase. After the bubbles disappear, stop heating for 1.0-1.5 hours, and then quickly cool down to 20-30°C for the next reaction;
[0054] Step 2):
[0055] Under the protection of nitrogen, add 0.1mol pentadecyl phosphate and 100mL tetrahydrofuran into a 500mL dry three-necked flask, cool down to about -20°C, add the dimethyl sulfoxide sodium salt solution prepared above dropwise, the dropwise addition process Control the internal temperature not to exceed -5°C, complete the dropwise addition in about 15 minutes, and...
Embodiment 2
[0061] A preparation method of β-Apo-12'-carotaldehyde, comprising the following steps:
[0062] step 1):
[0063] Under the protection of nitrogen, add 0.15 mol of sodium hydride solid into a 500mL reaction bottle, and slowly add 0.5 mol of dimethyl sulfoxide dropwise under magnetic mechanical stirring. After about 30 minutes, the dropwise addition is completed, and the internal temperature rises to 60-65°C for reaction. , the bubbles in the system gradually increased, and after the bubbles disappeared, stop heating, take 1.0-1.5h, and quickly cool down to 20-30°C for the next reaction;
[0064] Step 2):
[0065] Under the protection of nitrogen, add 0.1mol pentadecyl phosphate and 100mL tetrahydrofuran into a 500mL dry three-necked flask, cool down to -20°C, add the above-prepared dimethyl sulfoxide sodium salt solution dropwise, and the dropwise addition process is controlled The internal temperature does not exceed -5°C, the dropwise addition is completed in 15 minutes, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com