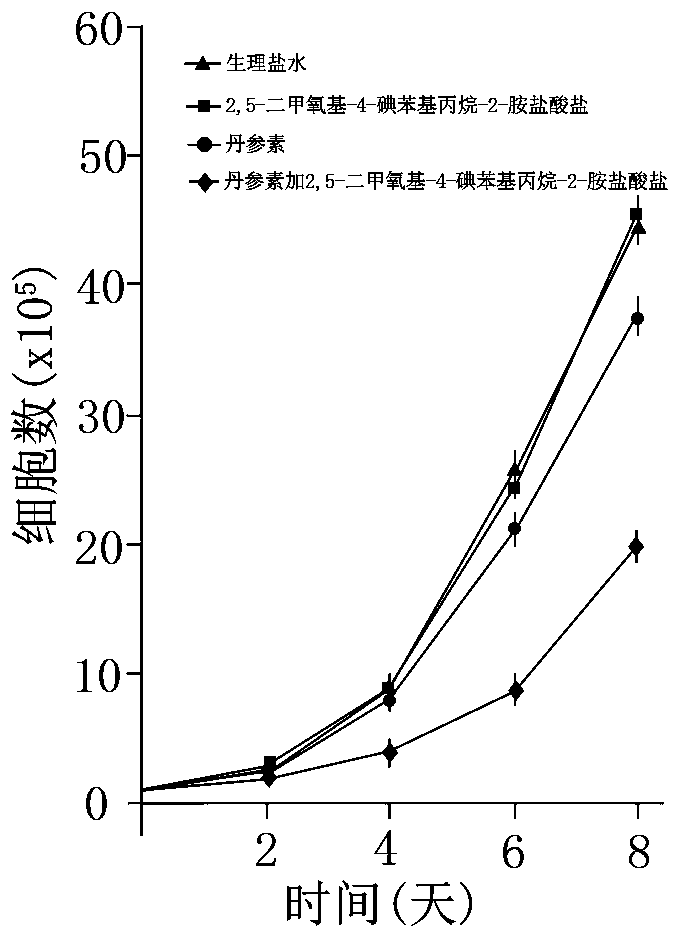
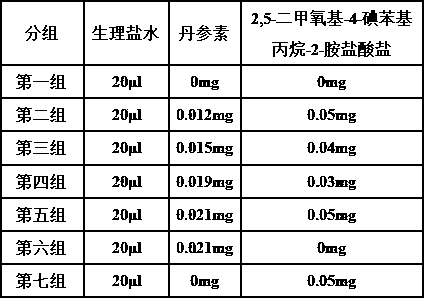
Intratumoral injection for treating rhabdomyosarcoma
A technique for rhabdomyosarcoma and injection, applied in the field of intratumoral injection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Intratumoral injection for the treatment of rhabdomyosarcoma and its preparation
[0026]
[0027] Preparation:
[0028] Mix danshensu and 2,5-dimethoxy-4-iodophenylpropane-2-amine hydrochloride, add water for injection, stir and dissolve at 20℃~37℃; then add mannitol and sodium acetate, 20℃ Stir and dissolve at ~37°C; filter through a 0.22-micron microporous membrane until clarified, and dispense into ampoules. Wherein the dosage of mannitol is 3 times of the weight of Danshensu, the consumption of sodium acetate is 0.06 times of the weight of Danshensu, and the dosage of 2,5-dimethoxy-4-iodophenylpropane-2-amine hydrochloride in the injection The concentration is 9.6mg / ml.
Embodiment 2
[0029] Example 2 Intratumoral injection for treating rhabdomyosarcoma and its preparation
[0030]
[0031] Preparation:
[0032] Mix danshensu and 2,5-dimethoxy-4-iodophenylpropane-2-amine hydrochloride, add water for injection, stir and dissolve at 20℃~37℃; then add mannitol and sodium acetate, 20℃ Stir and dissolve at ~37°C; filter through a 0.22-micron microporous membrane until clarified, and dispense into ampoules. Wherein the dosage of mannitol is 3 times of the weight of Danshensu, the consumption of sodium acetate is 0.06 times of the weight of Danshensu, and the dosage of 2,5-dimethoxy-4-iodophenylpropane-2-amine hydrochloride in the injection The concentration is 9.8mg / ml.
Embodiment 3
[0033] Example 3 Intratumoral injection for treating rhabdomyosarcoma and its preparation
[0034]
[0035] Preparation:
[0036] Mix danshensu and 2,5-dimethoxy-4-iodophenylpropane-2-amine hydrochloride, add water for injection, stir and dissolve at 20℃~37℃; then add mannitol and sodium acetate, 20℃ Stir and dissolve at ~37°C; filter through a 0.22-micron microporous membrane until clarified, and dispense into ampoules. Wherein the consumption of mannitol is 4 times of the weight of Danshensu, the consumption of sodium acetate is 0.05 times of the weight of Danshensu, 2,5-dimethoxy-4-iodophenylpropane-2-amine hydrochloride in the injection The concentration is 10.0mg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com