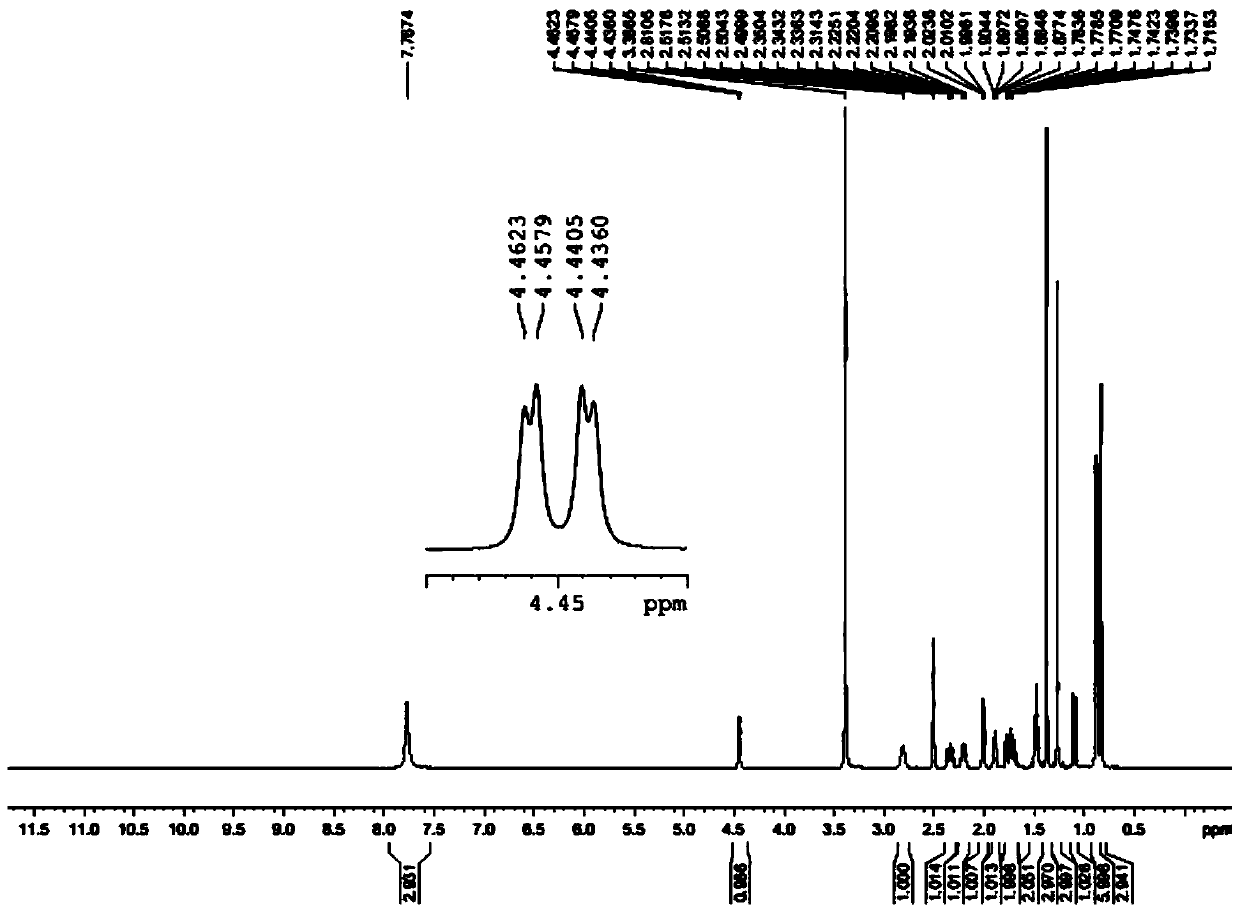
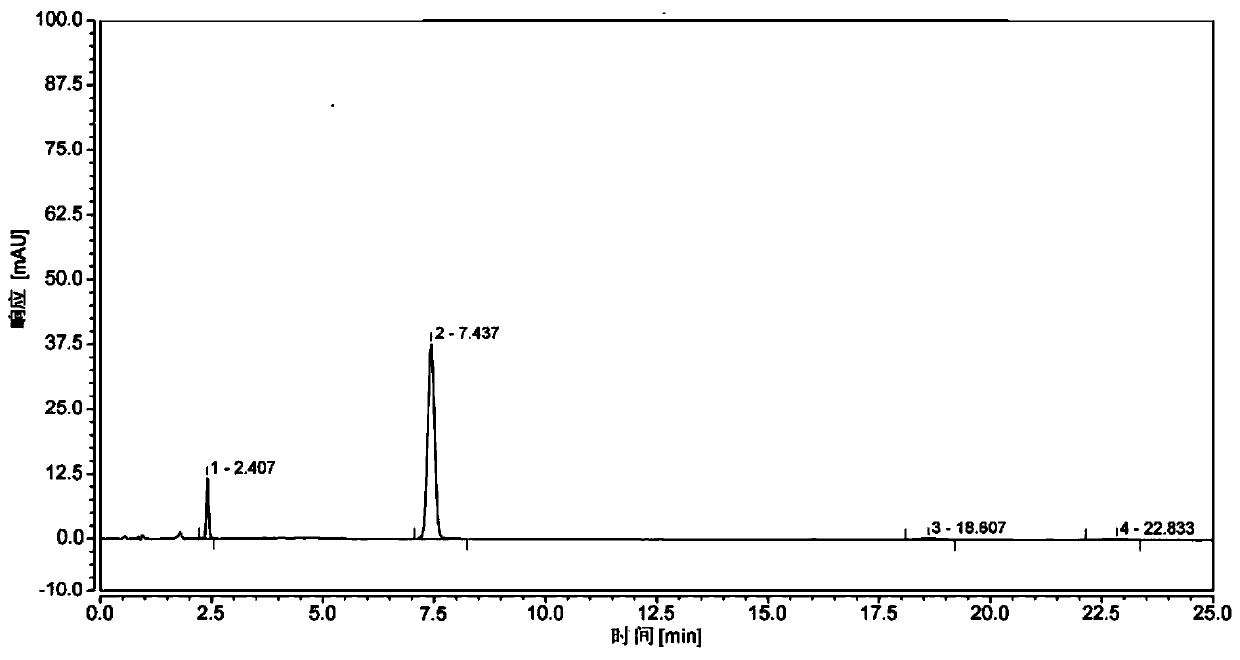
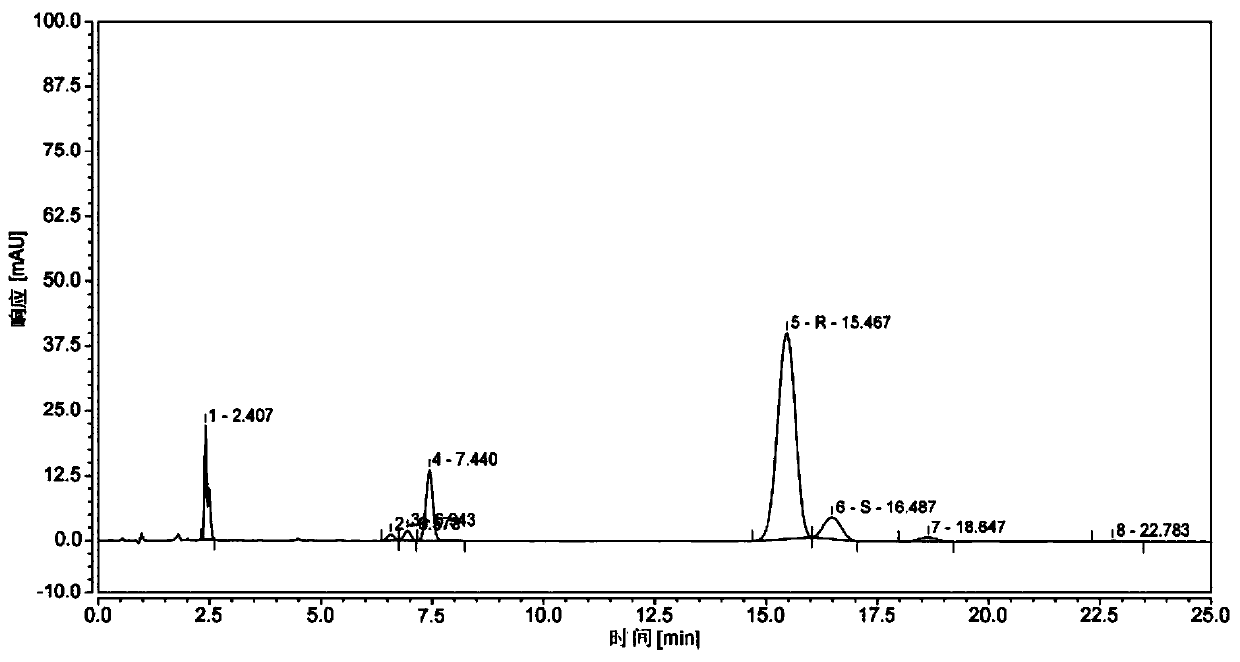
A kind of synthetic method of (r)-(1-amino-3-methyl)butyl-1-boronic acid pinanediol ester
A technology of pinanediol borate and synthesis method, which is applied in the field of medicine and chemical industry, can solve the problems of high price, harsh conditions, and high cost, and achieve the effects of being beneficial to industrial production, mild reaction conditions, and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (R)-Benzyl-(3-methyl-1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butane)carbamate Synthesis of Esters (Formula III)
[0049] Add (R)-1-amino-3-methylbutane-1-boronic acid pinacol ester hydrochloride (10mmol, 2.5g, formula II) and dichloromethane (30mL) into the there-necked flask, stir and wash with ice Cool in a water bath. Triethylamine (25mmol, 3.5mL) was added thereto, and stirred for 5-10min under an ice-water bath. A dichloromethane solution (10 ml) of benzyl chloroformate (11 mmol, 1.6 mL) was added slowly while keeping the temperature of the reaction mixture below 15° C. After the addition was complete, the reaction was continued at room temperature for 1 hour. Then add 1N dilute hydrochloric acid (10mL) under ice-water bath to quench the reaction, separate the organic layer, extract the aqueous layer with dichloromethane (2×30mL), combine the organic layers, wash with water (30mL), dry over anhydrous sodium sulfate, and filter , and concentrated to drynes...
Embodiment 2
[0059] (R)-tert-butyl-(3-methyl-1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butane)aminomethyl Synthesis of esters (formula III)
[0060] Add (R)-1-amino-3-methylbutane-1-boronic acid pinacol ester hydrochloride (10mmol, 2.5g, formula II) and dichloromethane (30mL) into the there-necked flask, stir and wash with ice Cool in a water bath. Triethylamine (25mmol, 3.5mL) was added thereto, and stirred for 5-10min under an ice-water bath. A dichloromethane solution (10 ml) of di-tert-butyl dicarbonate (11 mmol, 2.4 g) was added slowly while keeping the temperature of the reaction mixture below 15° C. After the addition, the temperature was raised to room temperature to continue the reaction for 1 hour. Then add 1N dilute hydrochloric acid (10mL) under ice-water bath to quench the reaction, separate the organic layer, extract the aqueous layer with dichloromethane (2×30mL), combine the organic layers, wash with water (30mL), dry over anhydrous sodium sulfate, and filter , and ...
Embodiment 3
[0067] (R)-N-(3-methyl-1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)butane)acetamide (formula III) Synthesis
[0068] Add (R)-1-amino-3-methylbutane-1-boronic acid pinacol ester hydrochloride (10mmol, 2.5g, formula II) and dichloromethane (30mL) into the there-necked flask, stir and wash with ice Cool in a water bath. Triethylamine (25mmol, 3.5mL) was added thereto, and stirred for 5-10min under an ice-water bath. A dichloromethane solution (10 ml) of acetic anhydride (11 mmol, 1.1 g) was added slowly while keeping the temperature of the reaction mixture below 15° C. After the addition, the temperature was raised to room temperature to continue the reaction for 1 hour. Then add 1N dilute hydrochloric acid (10mL) under ice-water bath to quench the reaction, separate the organic layer, extract the aqueous layer with dichloromethane (2×30mL), combine the organic layers, wash with water (30mL), dry over anhydrous sodium sulfate, and filter , and concentrated to dryness under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com