Systems, devices, and methods for episode detection and evaluation
A symptom, monitoring system technology, applied in the direction of error detection/correction, application, telemetry patient monitoring, etc., can solve the problems of confusing users, blows, and unclear patients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
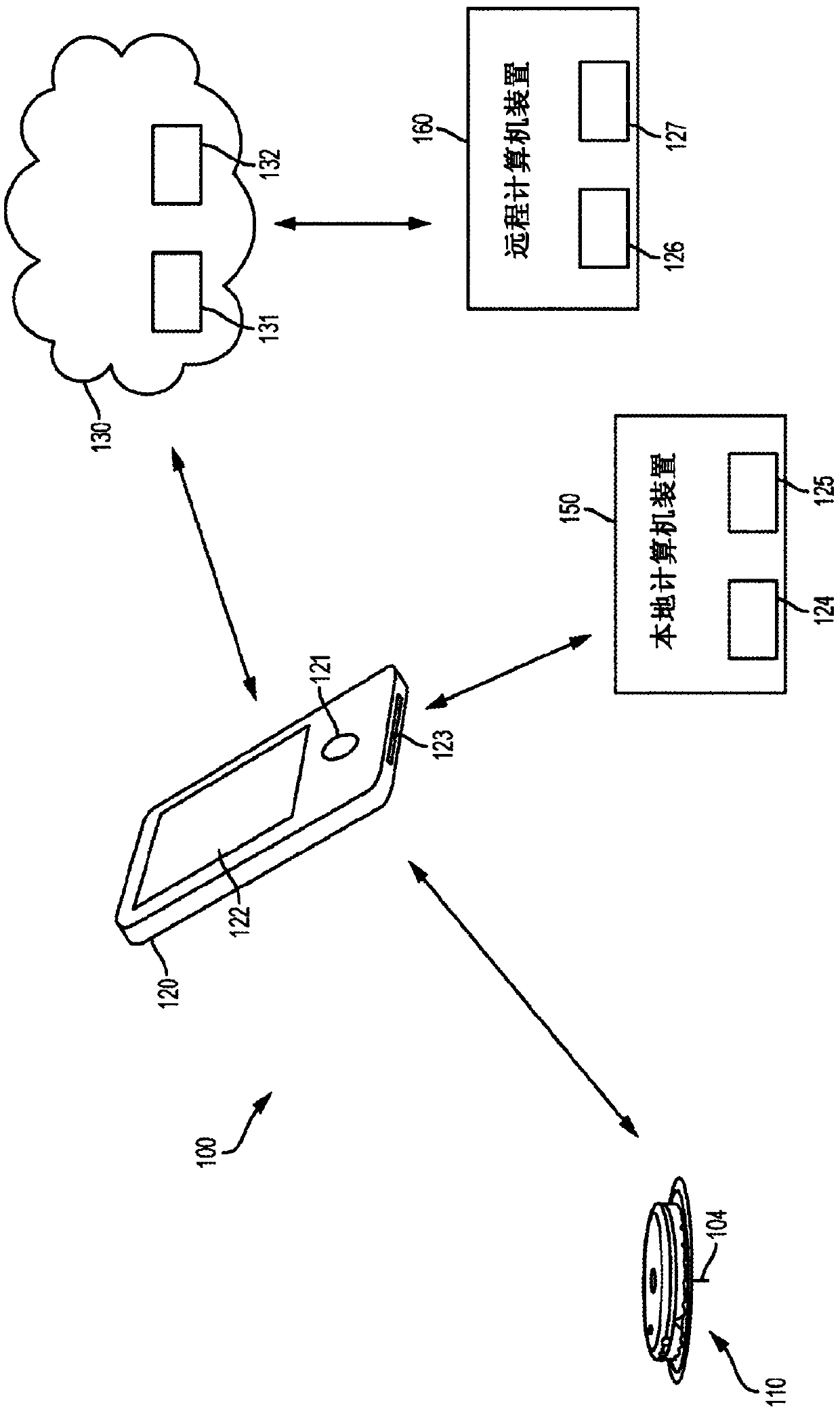
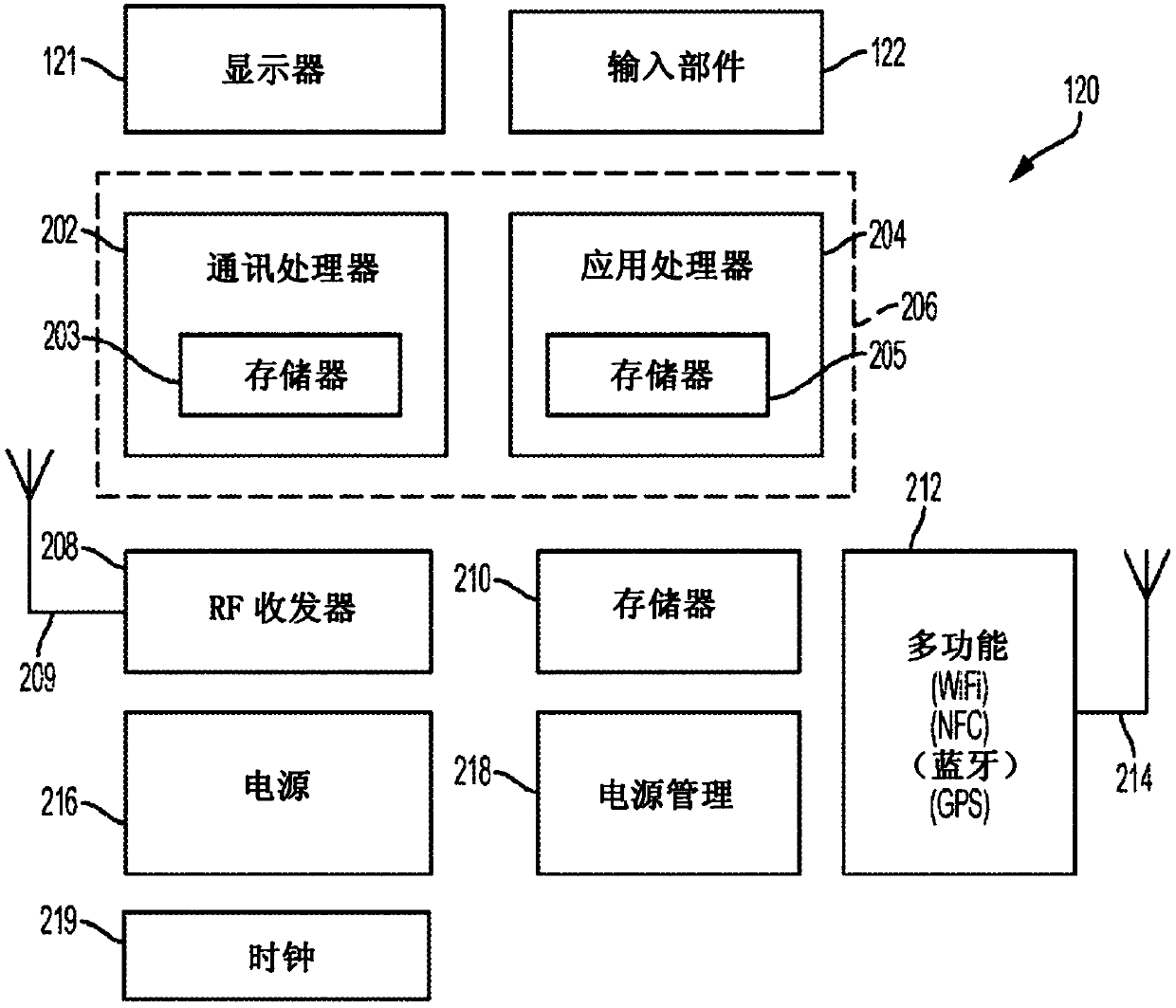
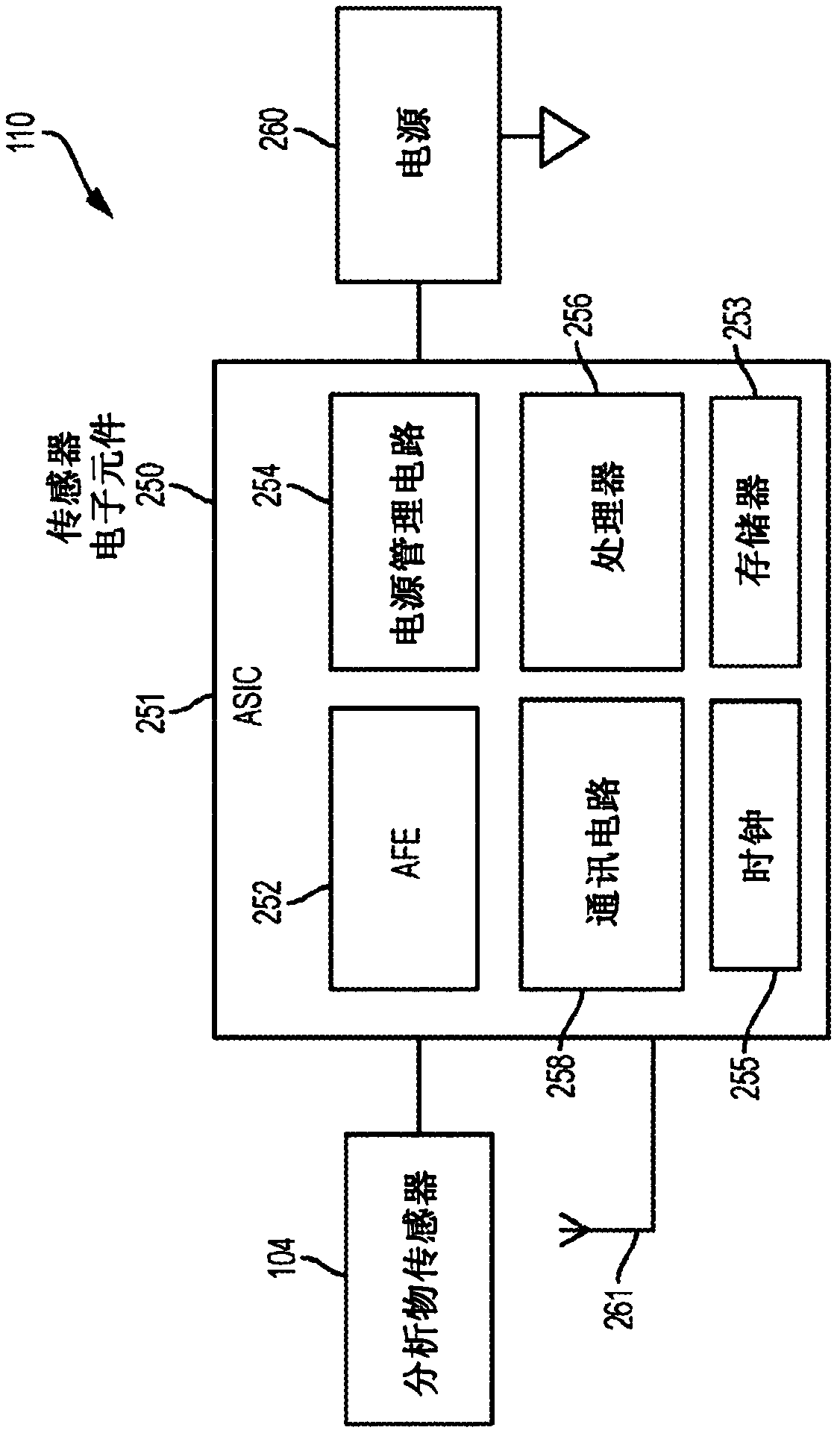
[0059] EIS can be used to detect previous, current and / or future symptom onset. As used herein, the term symptom onset does not mean every change in analyte level, but rather those changes that contribute significantly to the subject's analyte variability, such as high glucose and low glucose spikes. Symptomatic episodes can also include clinically significant glucose excursions or events, such as rapid increases or rapid decreases in glucose levels. Different criteria can be used to define a set of analyte level measures as a symptom onset, including the magnitude of an analyte measure, the rate of change between consecutive analyte measures or other sets of measures in close time, the analyte in which a threshold level is exceeded Number of measurements (or durations), breakthrough of a threshold by integrated area of a series of analyte measurements, any combination thereof, etc. The detection conditions for these onset of symptoms are described in US Patent Publication ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com