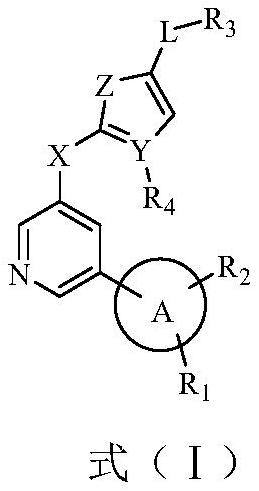
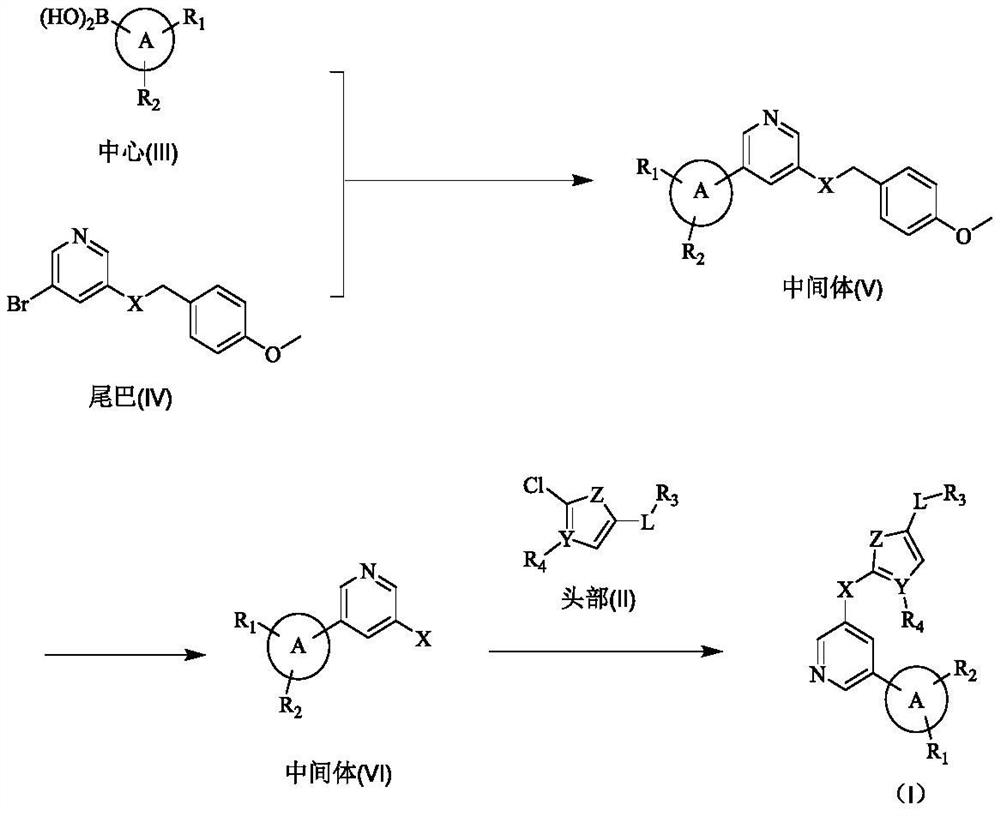
A class of biarylpyridine deubiquitinase inhibitors, its preparation method and application
A pyridyl and halogen technology, applied in organic chemistry, pharmaceutical formulation, bulk chemical production, etc., can solve the problems of unsatisfactory micromolar level specificity and low efficiency, and achieve reasonable route design, simple operation and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
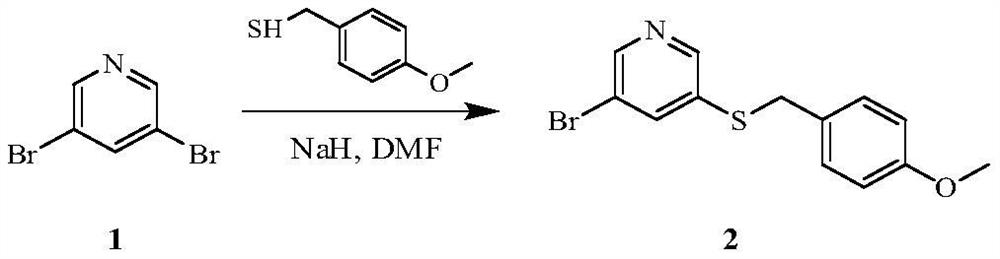
[0103] Example 1 3-bromo-5-(4-methoxybenzylthio)pyridine
[0104]
[0105] 4-Methoxybenzylthiol (32.55g, 211.07mmol) was dissolved in 500mL dimethylformamide, NaH (9.29g, 232.18mmol) was added, and reacted at 20°C for 30min, then, 3,5-dibromopyridine ( 1,50.00g, 211.07mmol), react at 20°C for 16 hours, add 100mL of water to terminate the reaction, extract with ethyl acetate (100mL x 3), wash the organic layer with water (200mL x 3), dry over anhydrous sodium sulfate, and recover under reduced pressure The solvent was separated by column chromatography to obtain 48.0 g of white solid (compound 2), with a yield of 73%. 1 HNMR (400MHz, CDCl 3 ): δ=8.38(d, J=2.1Hz, 1H), 8.31(d, J=1.9Hz, 1H), 7.60(t, J=2.0Hz, 1H), 7.13-7.08(m, 2H, Ar- H),6.79-6.73(m,2H,Ar-H),4.00(s,2H,CH 2 ),3.71(s,3H,CH 3 ).ESI-MS: m / z=311[M+H] + .
Embodiment 2
[0106] Example 2 3-(2-isopropylphenyl)-5-(4-methoxybenzylthio)pyridine
[0107]
[0108] Compound 2 (15.00g, 48.35mmol), 2-isopropylphenylboronic acid (8.72g, 53.19mmol), Pd(dppf)Cl 2 (3.54g, 4.84mmol) and sodium carbonate (10.25g, 96.71mmol) were added to 150mL of dioxane / water (10 / 1), stirred and reacted at 100°C for 16 hours, and the solvent was recovered under reduced pressure after the reaction, and 20mL of water was added , extracted with dichloromethane (50mL x 3), dried over anhydrous sodium sulfate, recovered the solvent under reduced pressure, and obtained 13.6g of yellow oil (compound 3) by column chromatography with a yield of 78%. 1 H NMR (400MHz, CDCl 3 ): δ=8.52(d, J=2.2Hz, 1H, pyridine-H), 8.36(d, J=1.9Hz, 1H, pyridine-H), 7.48(t, J=2.1Hz, 1H, pyridine-H ),7.42-7.35(m,2H,Ar-H),7.25-7.16(m,2H,Ar-H),7.11-7.02(m,1H,Ar-H),6.81(d,J=8.6Hz, 2H,Ar-H),4.10(s,2H,CH 2 ),3.78(s,3H,CH 3 ),2.86(q,J=13.7,6.8Hz,1H,CH),1.15(t,J=10.8Hz,6H,CH 3 ).ESI-MS: m / z=359.5[M+H] ...
Embodiment 3
[0109] Example 3 3-benzyl-5-(4-methoxybenzylthio)pyridine
[0110]
[0111] Synthesis of Compound 4: Using Compound 2 and 2-ethylphenylboronic acid as raw materials, the synthesis and post-treatment were the same as in Example 2. 11.7 g of a yellow oil (compound 4) was obtained, yield 72%. 1 H NMR (400MHz, CDCl 3): δ=8.52(d, J=2.1Hz, 1H, pyridine-H), 8.38(d, J=1.8Hz, 1H, pyridine-H), 7.50(t, J=2.0Hz, 1H, pyridine-H ),7.39–7.15(m,5H,Ar-H),7.10(d,J=7.7Hz,1H,Ar-H),6.81(d,J=8.6Hz,2H,Ar-H),4.11(d ,J=7.4Hz,2H,CH2),3.78(s,3H,CH3),2.50(q,J=7.5Hz,2H,CH2),1.07(t,J=7.5Hz,3H,CH3).ESI- MS:m / z=336.5[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com