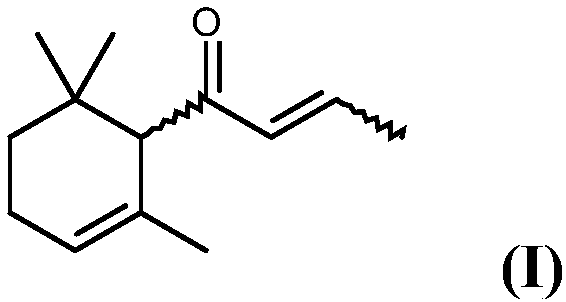
Process for preparing alpha-damascone
一种三烯、二甲基十一的技术,应用在制备ɑ‑突厥酮领域,能够解决降低总收率、总收率不令人满意、不适合柠檬醛有效生产α-突厥酮等问题,达到减少副产物的量、工艺适用性简单且便宜的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1: Synthesis of 6,10-dimethylundec-1,5,9-trien-4-ol from citral (step a)
[0148] Add allyl magnesium chloride (362.6ml, 2M in THF, 0.72mol) dropwise to a stirred solution of citral (100g, 0.65mol) in dry THF (750ml) while keeping the temperature between 0°C and 5°C . After the allyl magnesium chloride was completely added, the reaction mixture was stirred at 0°C for 1 hour, and the progress of the reaction was monitored by TLC. After the reaction was completed, the mixture was quenched by adding saturated ammonium chloride solution (500 ml) at 0-10°C. The aqueous layer was extracted with ethyl acetate (3×50 ml), and the combined organic layer was concentrated to obtain 127 g of compound 2 (99.5%).
[0149] 1 HNMR(300MHz, CDCl 3 )δ1.53(s,3H),1.61(s,6H),1.94-2.07(m,4H),2.18-2.23(m,2H),4.34-4.36(m,1H),5.01-5.14(m, 4H), 7.72-5.75 (m, 1H).
Embodiment 2
[0150] Example 2: Synthesis of 6,10-dimethylundec-1,5,9-trien-4-one using TEMPO / iron(III) nitrate nonahydrate / NaCl (step b)
[0151] Add iron (III) nitrate nonahydrate (2.0g, 5.1mmol, 0.1 equivalent), TEMPO (0.79g, 5.1mmol, 0.1 equivalent) and solid NaCl (0.29g, 51mmol, 0.1 equivalent) to 50ml of toluene at 25°C . The 6,10-dimethylundec-1,5,9-trien-4-one (10.0 g, 51 mmol, 1 equivalent) obtained in Example 1 was added to the mixture at 25° C. Stir for 3 hours under oxygen atmosphere (O 2 The gas continuously passes through the reaction mixture). The reaction was monitored by TLC and HPLC, showing a conversion rate of 50%. After stirring for 3 hours, add an additional amount of iron (III) nitrate nonahydrate (2.0g, 5.1mmol, 0.1 equivalent), TEMPO (0.79g, 5.1mmol, 0.1 equivalent), NaCl (0.29g) at 25°C , 5.1 mmol, 0.1 equivalent) was added to the reaction mixture, and then the reaction mixture was stirred for another 2 hours. After consumption of almost 95% of the starting materi...
Embodiment 3
[0153] Example 3: Synthesis of 6,10-dimethylundec-1,5,9-trien-4-one using 4-OH TEMPO / iron(III) nitrate nonahydrate / NaCl (step b)
[0154] Add iron (III) nitrate nonahydrate (13.3g, 32.9mmol, 0.1 equivalent), 4-hydroxy TEMPO (5.7g, 32.9mmol, 0.1 equivalent) and solid NaCl (1.9g, 32.9 mmol, 0.1 equivalent). The 6,10-dimethylundec-1,5,9-trien-4-ol (64.0 g, 329.8 mmol, 1 equivalent) obtained in Example 1 was added to the mixture at 25°C and Stir for 3 hours at 45℃ under oxygen atmosphere (O 2 The gas continuously passes through the reaction mixture). The reaction was monitored by TLC and HPLC, showing a conversion rate of 50%. After stirring for 3 hours, add an additional amount of iron(III) nitrate nonahydrate (13.3g, 32.9mmol, 0.1 equivalent), 4-hydroxy TEMPO (5.7g, 32.9mmol, 0.1 equivalent) and NaCl at 45°C (1.9 g, 32.9 mmol, 0.1 equivalent) was added to the reaction mixture, and then the reaction mixture was stirred for another 2 hours. After consumption of almost 95% of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com