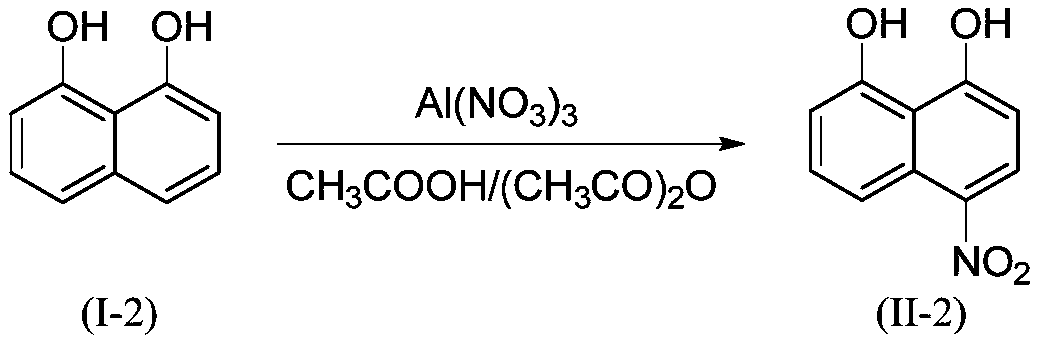Patents
Literature
51 results about "Nitronate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
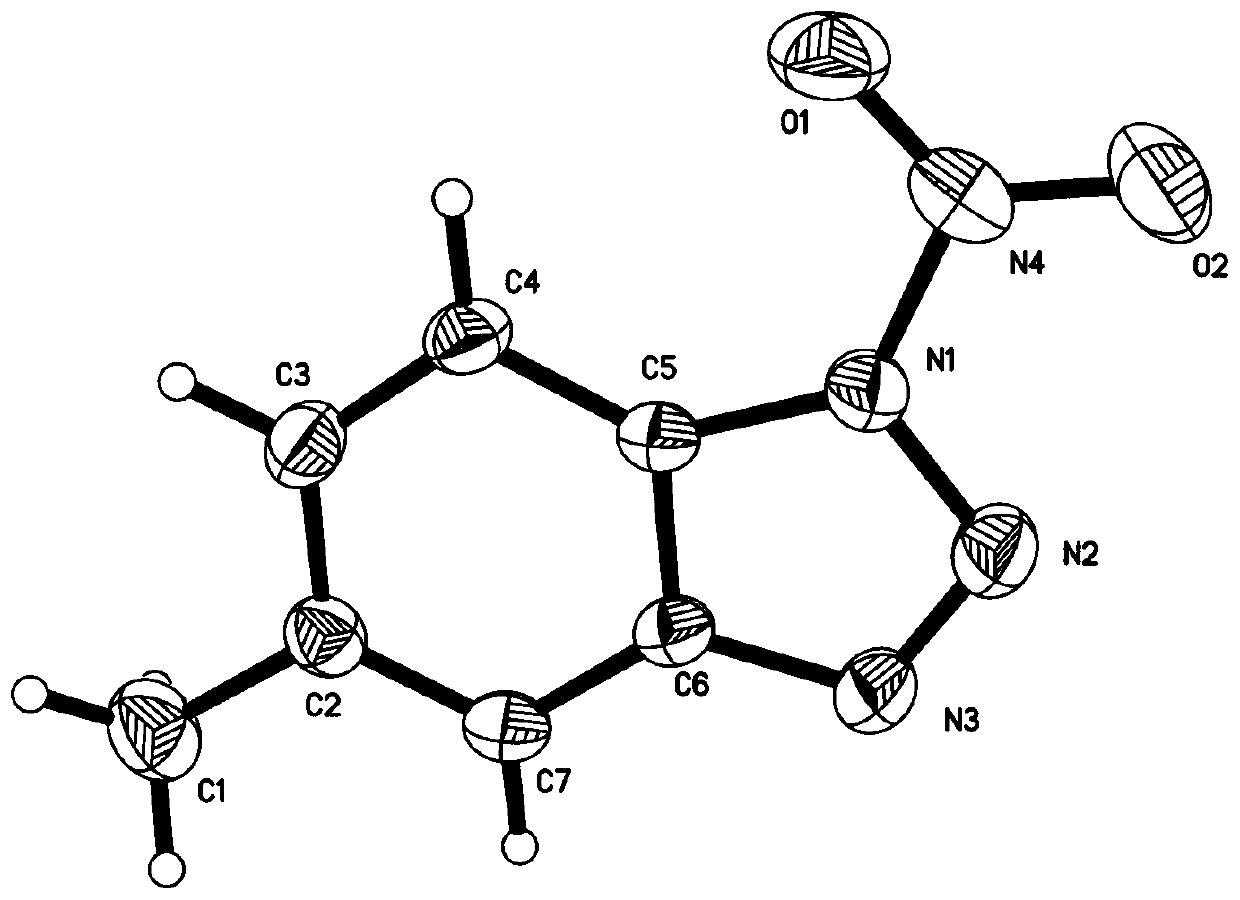
A nitronate (IUPAC: azinate) in organic chemistry is a functional group with the general structure R¹R²C=NO⁻ ₂. It is the anion of a nitronic acid, a tautomeric form of a nitro compound. Just as ketones and aldehydes exist in equilibrium with their enol tautomer in basic and acidic conditions, nitro compounds exist in equilibrium under basic conditions with their nitronate tautomer. The base deprotonates the α carbon, or the carbon directly attached to the nitrogen. The nitronate has two different resonance structures, one with a negative charge on the α carbon and a double bond between the nitrogen and one of the oxygens, and another resonance structure with a double bond between the nitrogen and the α carbon, and no double bond between the nitrogen and the oxygens. A nitronic acid is also called an aci form. In the Nef reaction nitronic acids are degraded to ketones. They can be alkylated on oxygen and used as a dipole in 1,3-dipolar cycloadditions.
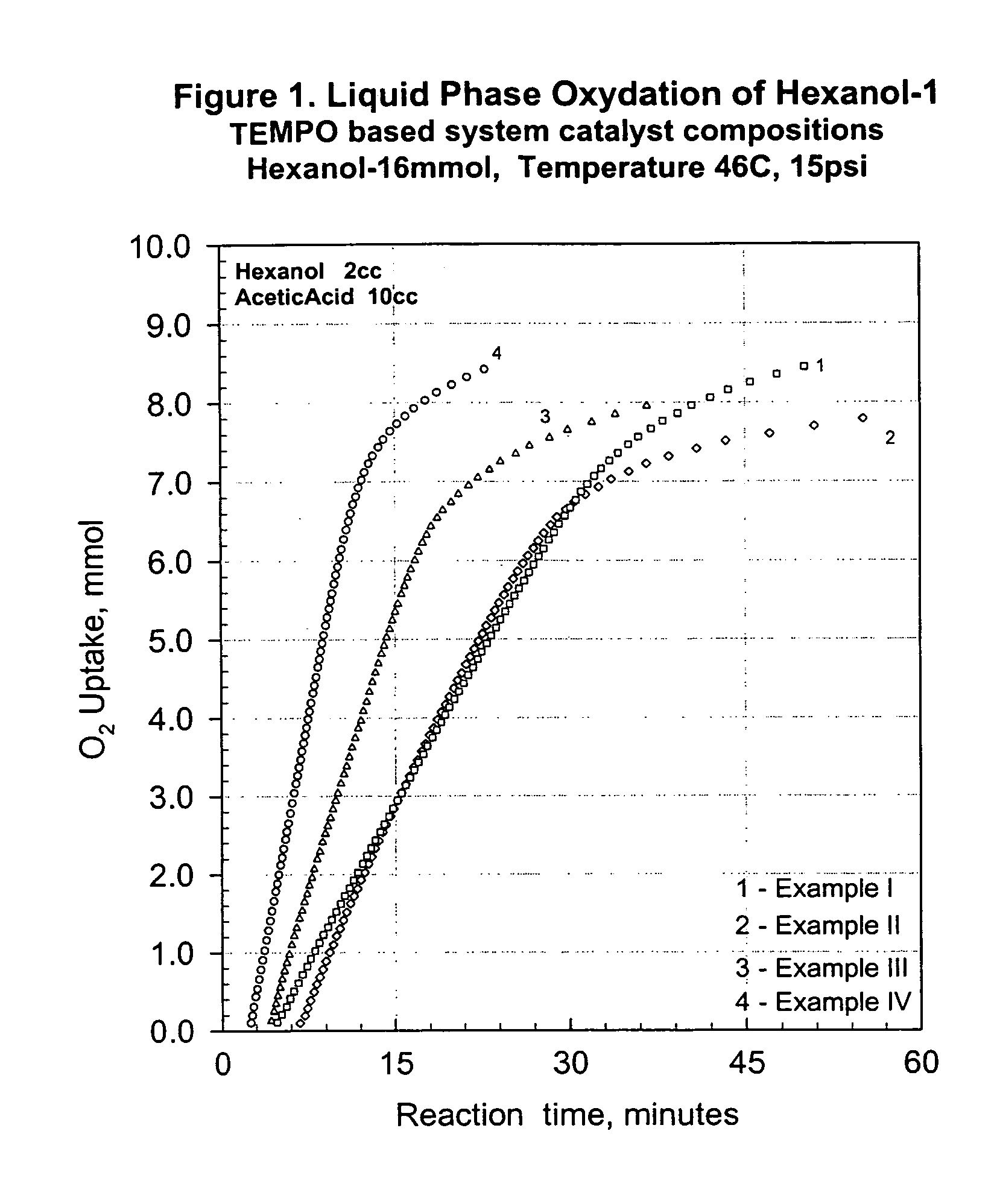
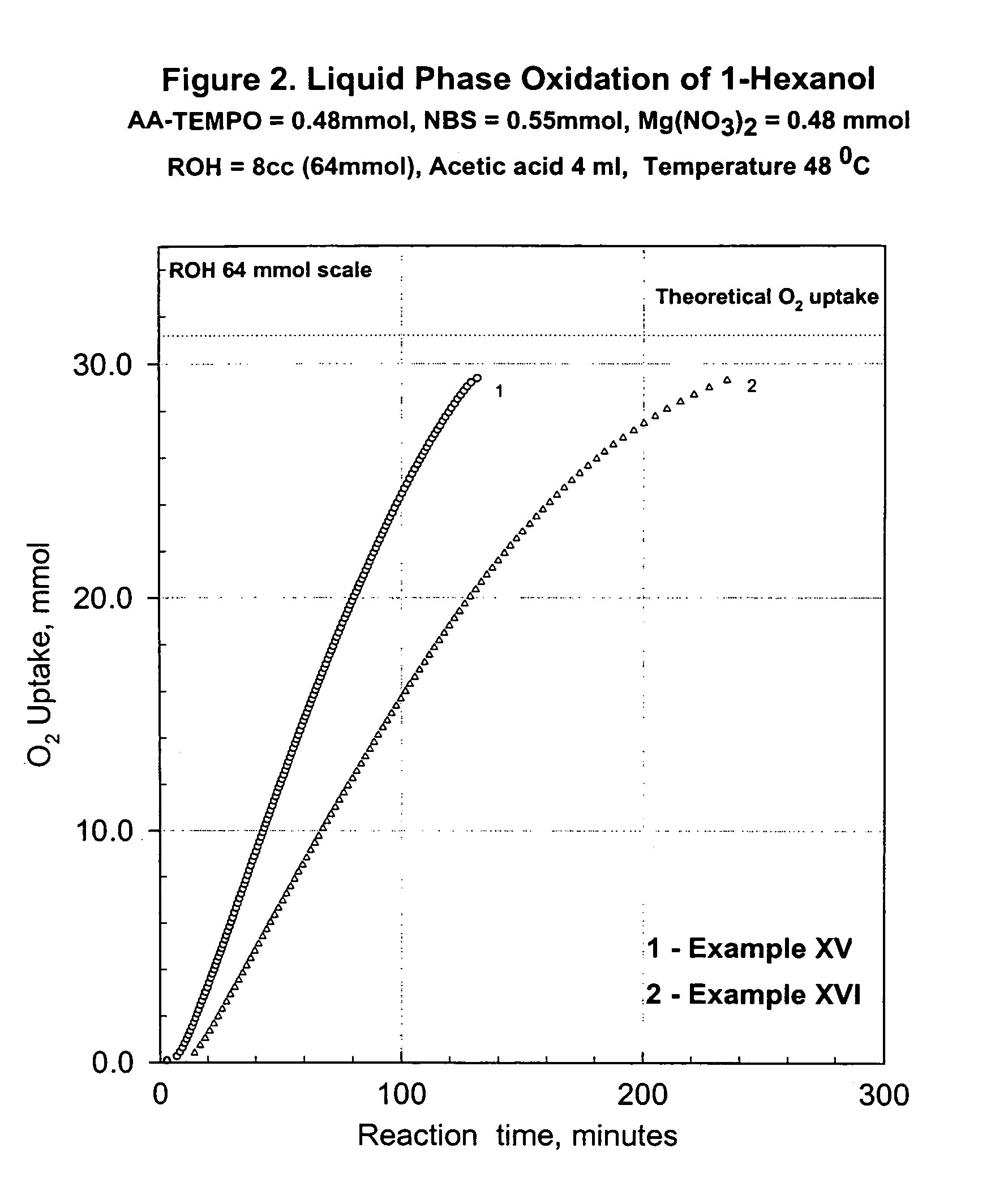
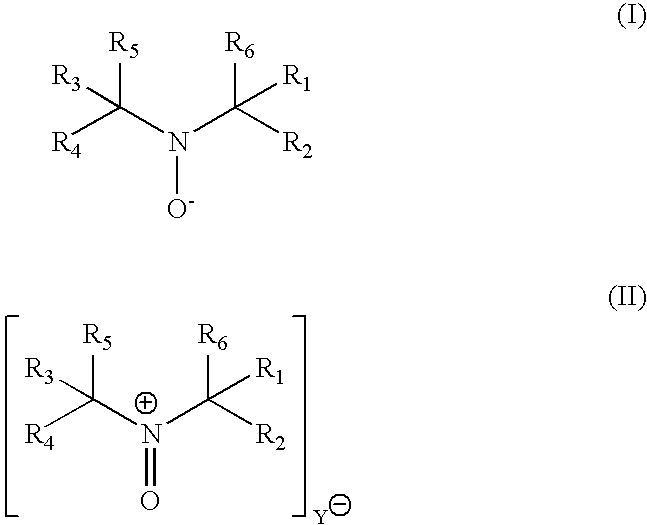
Process for transition metal free catalytic aerobic oxidation of alcohols under mild conditions using stable free nitroxyl radicals
InactiveUS7030279B1High selectivityHigh reaction rateOrganic compound preparationCarbonyl compound preparation by oxidationKetoneAldehyde
An alcohol can be oxidized by a process in which a primary or secondary alcohol are reacted with an oxygen-containing gas in the presence of a catalyst composition containing (i) a stable free nitroxyl radical derivative, (ii) a nitrate source, (iii) a bromide source, and (iiii) a carboxylic acid, thereby obtaining an aldehyde or a ketone.
Owner:EVONIK DEGUSSA GMBH
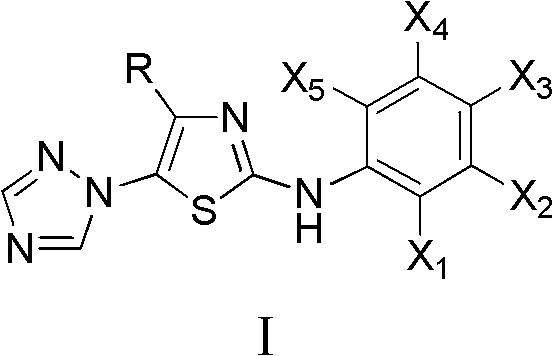
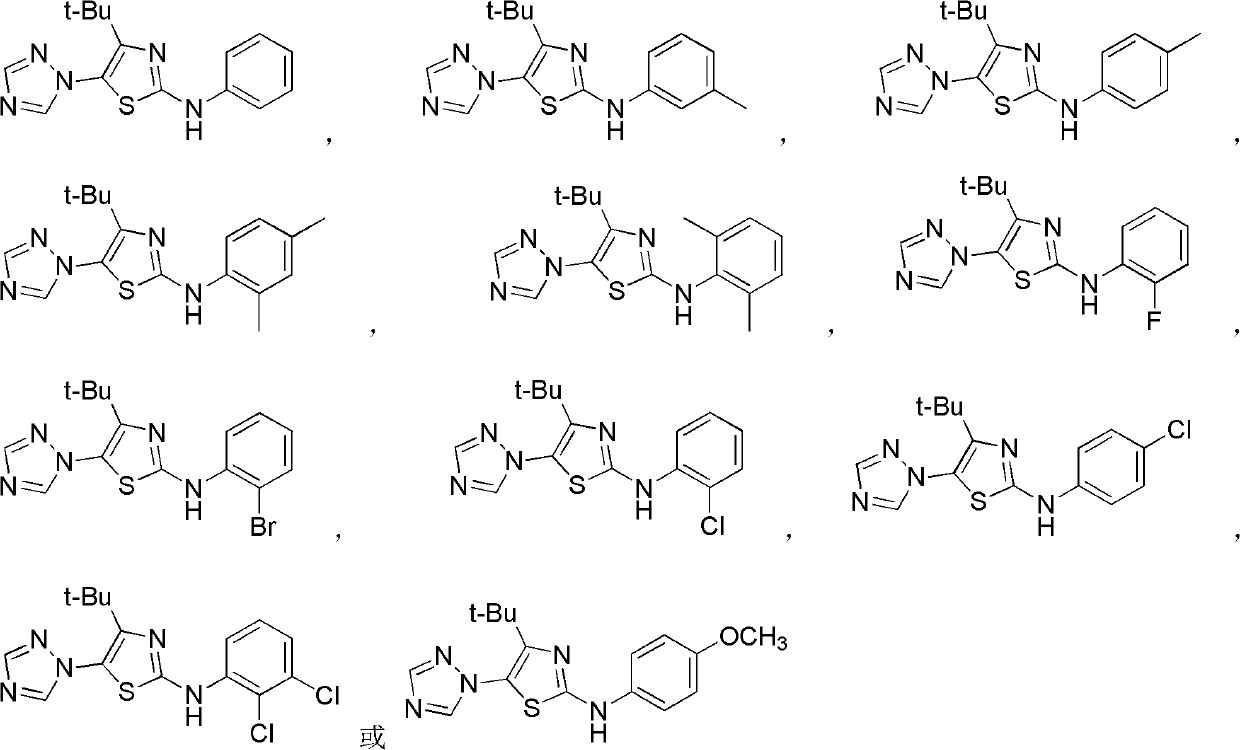
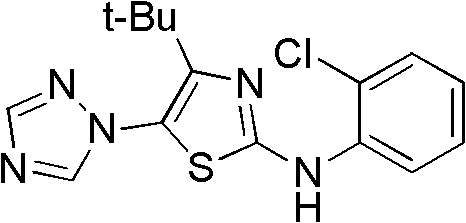
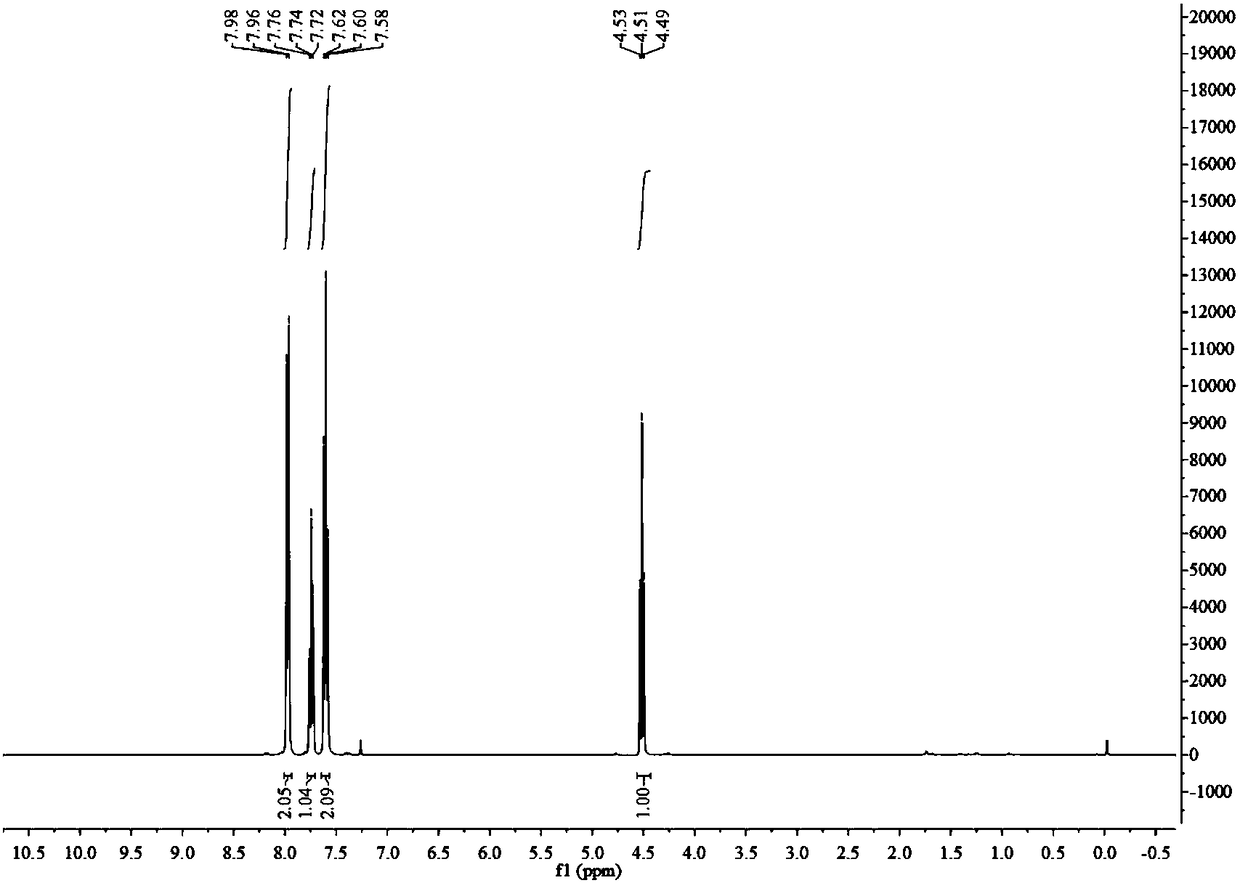
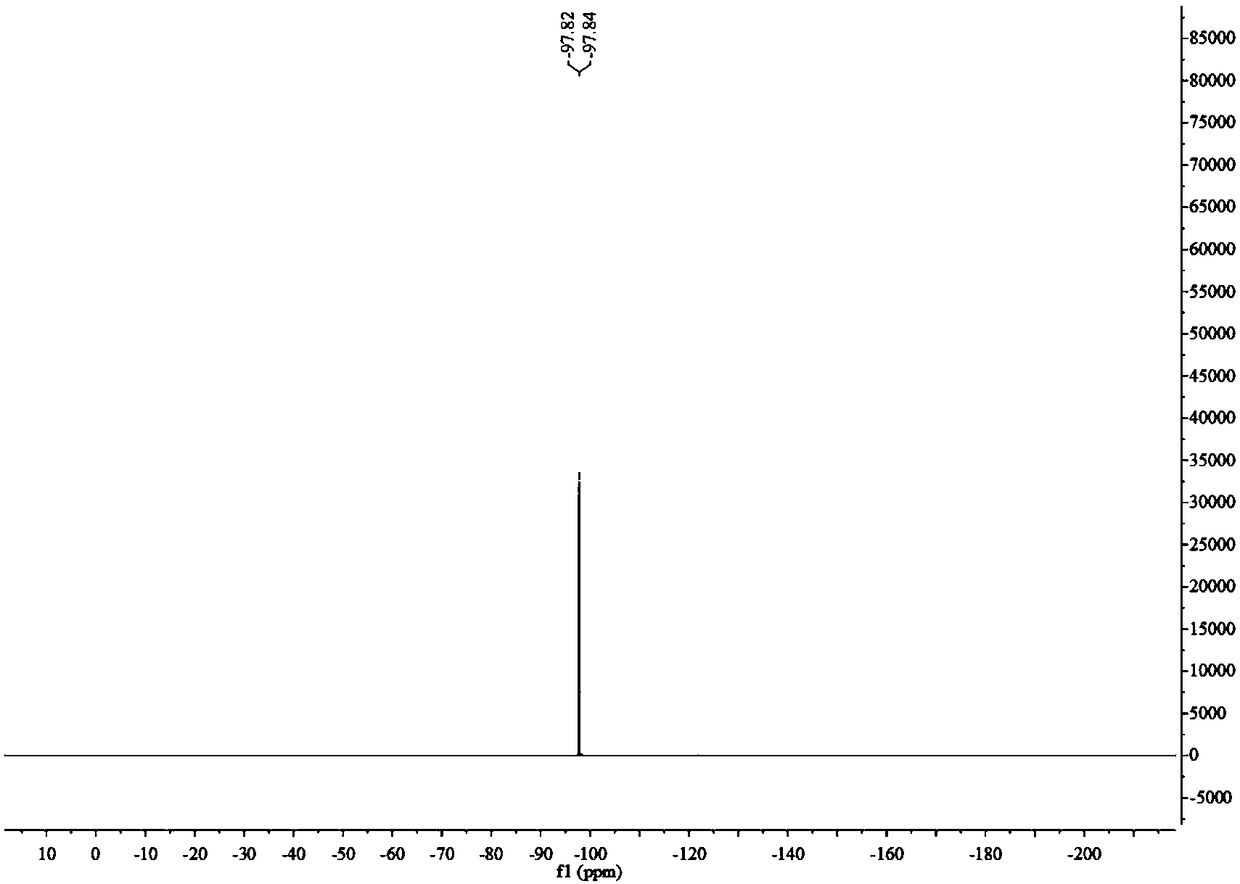
4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and application thereof to preparation of medicaments for resisting cancer
InactiveCN102675303AAnti-cervical cancerCtiveOrganic chemistryAntineoplastic agentsHydrobromidePhosphate
The invention discloses 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole and salts thereof shown in a formula I, wherein R is selected from H, alkyl groups of C1-C2 and straight-chain alkyl groups or branched-chain alkyl groups of C3-C4; X1, X2, X3, X4 and X5 are selected from hydrogen, alkyl groups of C1-C2, hydroxide group, methoxy group, oxyethyl group, trifluoromethyl, fluorine, chlorine, bromine, nitryl, amino group, acetyl amino group, methanesulfamide, ethoxycarbonyl or carboxyl; and the salts of the 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole are selected from hydrochloride, hydrobromide, nitrate, sulfate, phosphate, mesylate, p-toluene sulfonate, tartrate, lactate or malate. The 4-alkyl-2-arylamino-5-(1,2,4-triazole-1-group) thiazole is applied to preparation of medicaments for resisting cervical cancer or lung neoplasms.
Owner:HUNAN UNIV
Rapid detection method for nitrites in blood and urine
InactiveCN104280383APlay a separate rolePlay a role in high enrichmentMaterial analysis by observing effect on chemical indicatorCoupling reactionPharmacology
The invention discloses a rapid detection method for nitrites in blood and urine. The method comprises: utilizing a diazotization coupling reaction of nitrites and Griess reagent to form an azo compound, performing cloud point extraction, precipitating the colored azo compound at the bottom of a centrifuge tube, comparing the red color at the tube bottom with standard color gradation, and determining the content of the nitrite according to the color depth. Because the enriching multiple of cloud point extraction is large and blood and urine are subjected to decoloring and protein-removal processing, the interference is substantially eliminated, and the detection limit of nitrites in the system can reach 0.01 mu g / mL. The method has the characteristics of simpleness, rapidness, high sensitivity, on-site detection and the like.
Owner:云南健牛环境监测有限公司
Rapid detection method of nitrite in blood and urine with high sensitivity
InactiveCN104458712ALow cloud pointMild operating conditionsMaterial analysis by observing effect on chemical indicatorActive agentDioxyethylene Ether
The invention discloses a rapid detection method of nitrite in blood and urine with high sensitivity. Azo compound is formed through diazotization coupling reaction by nitrite and a Griess agent. A nonionic surfactant including fatty alcohol polyoxyethylene ether AEO series is used in cloud point extraction. The method has advantages such as mild operation condition, low cloud point temperature, short phase-separation time, high extraction efficiency and the like. The detection limit of nitrite in the system can reach 0.005 micrograms / mL. According to the invention, the method with high sensitivity is simple and fast and can be used on site.
Owner:云南健牛环境监测有限公司
2-arylsulfonyl-2, 2-difluorodiazoethane compound, preparation method and application thereof
InactiveCN108383761AEasy to manufactureEasy to useOrganic chemistryOrganic compound preparationCycloadditionAlkyne
The invention relates to a 2-arylsulfonyl-2, 2-difluorodiazoethane compound, a preparation method and application thereof. The preparation method of the 2-arylsulfonyl-2, 2-difluorodiazoethane compound includes: dissolving 2, 2-difluoro-2-aryl sulfonyl ethylamine hydrochloride in a mixed solvent of an organic solvent and water, adding nitrite, carrying out stirring at room temperature for completereaction, and then conducting washing, extraction, separation and column chromatography purification to obtain the yellow liquid 2-arylsulfonyl-2, 2-difluorodiazoethane. The obtained target compoundis a diazo compound containing sulfonyl difluoromethyl, can be subjected to [3+2] cycloaddition reaction with alkyne so as to synthesize a pyrazole compound containing difluoromethyl. The difluorodiazoethane compound provided by the invention has the characteristics of convenient preparation, simple use and mild reaction conditions, and the invention provides an effective method for synthesis of compounds containing difluoromethyl.
Owner:TIANJIN UNIV
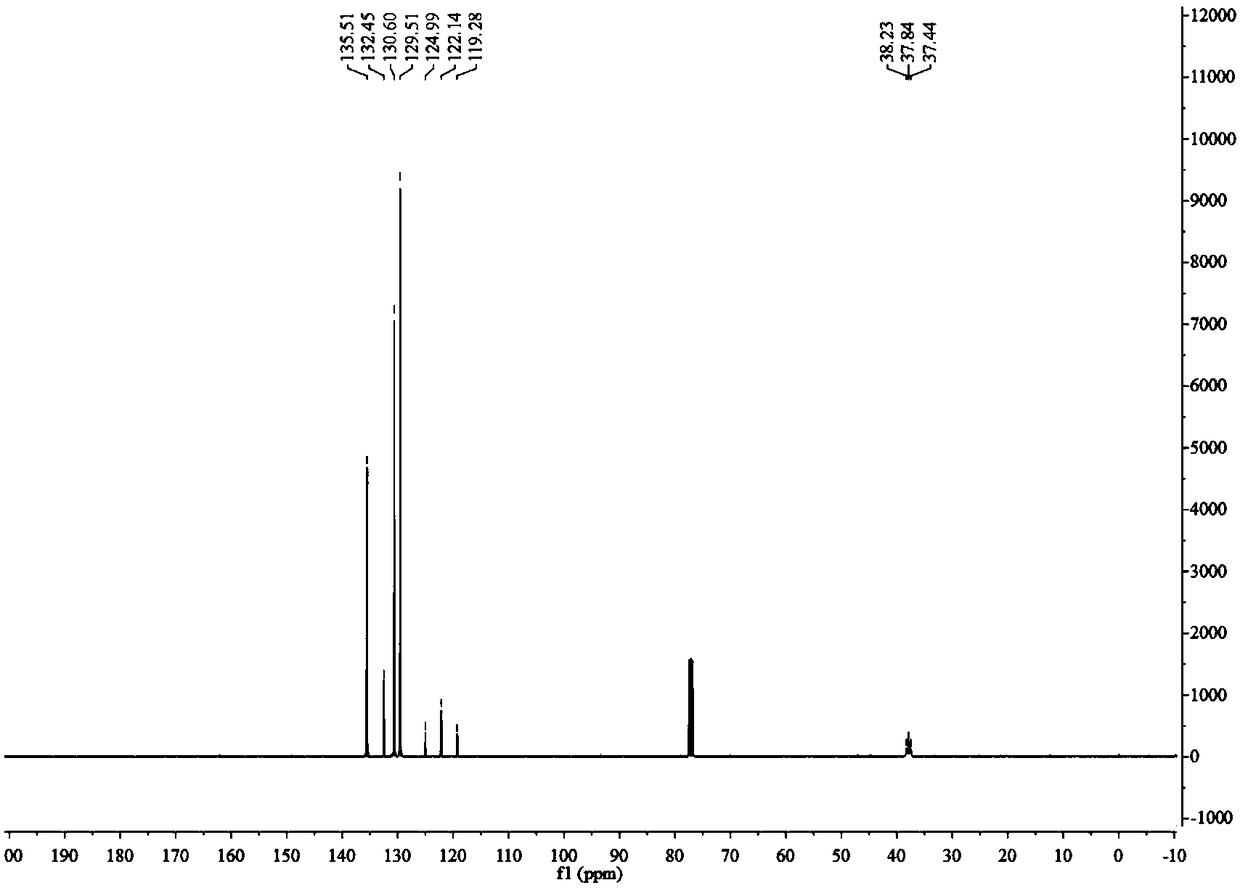
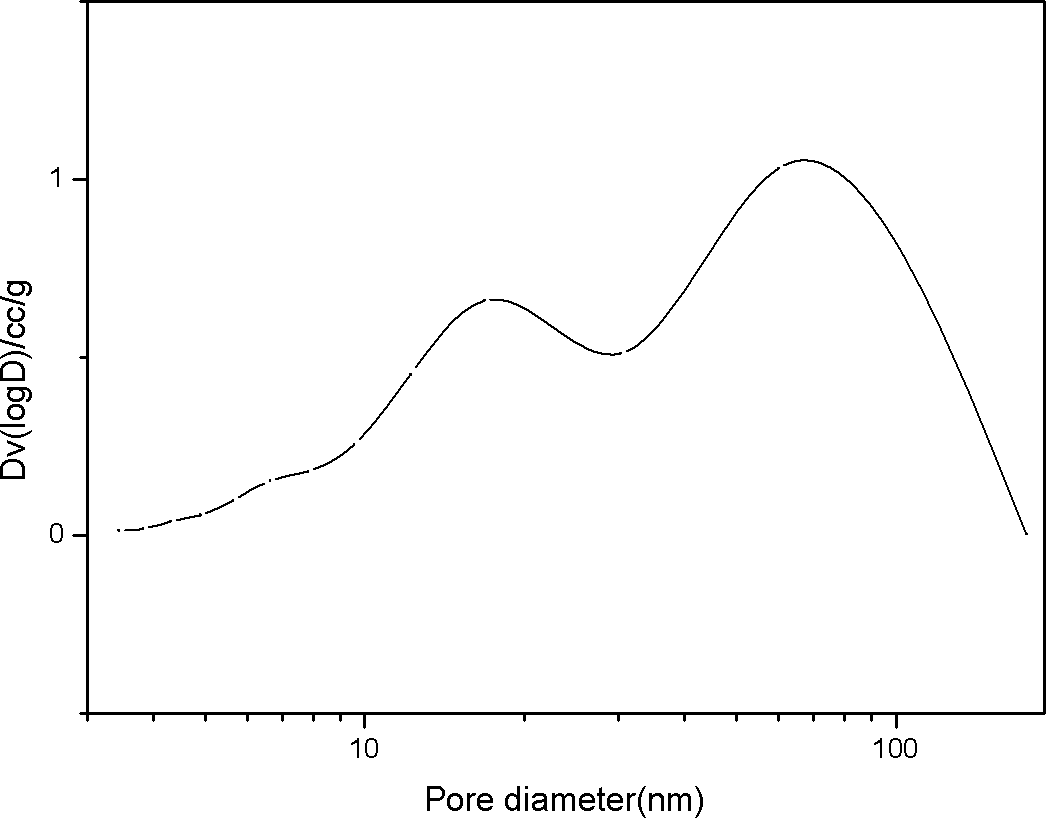
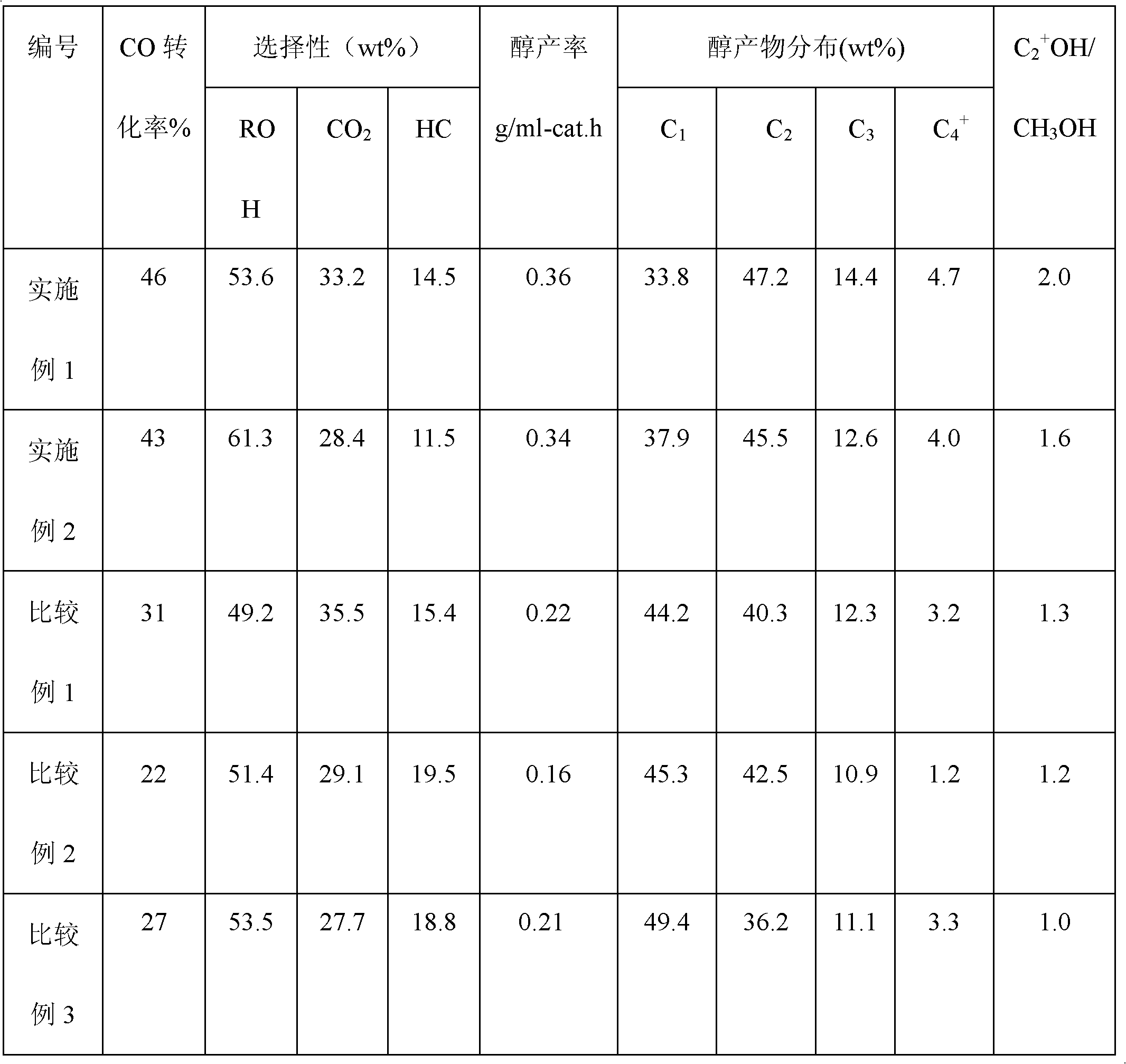
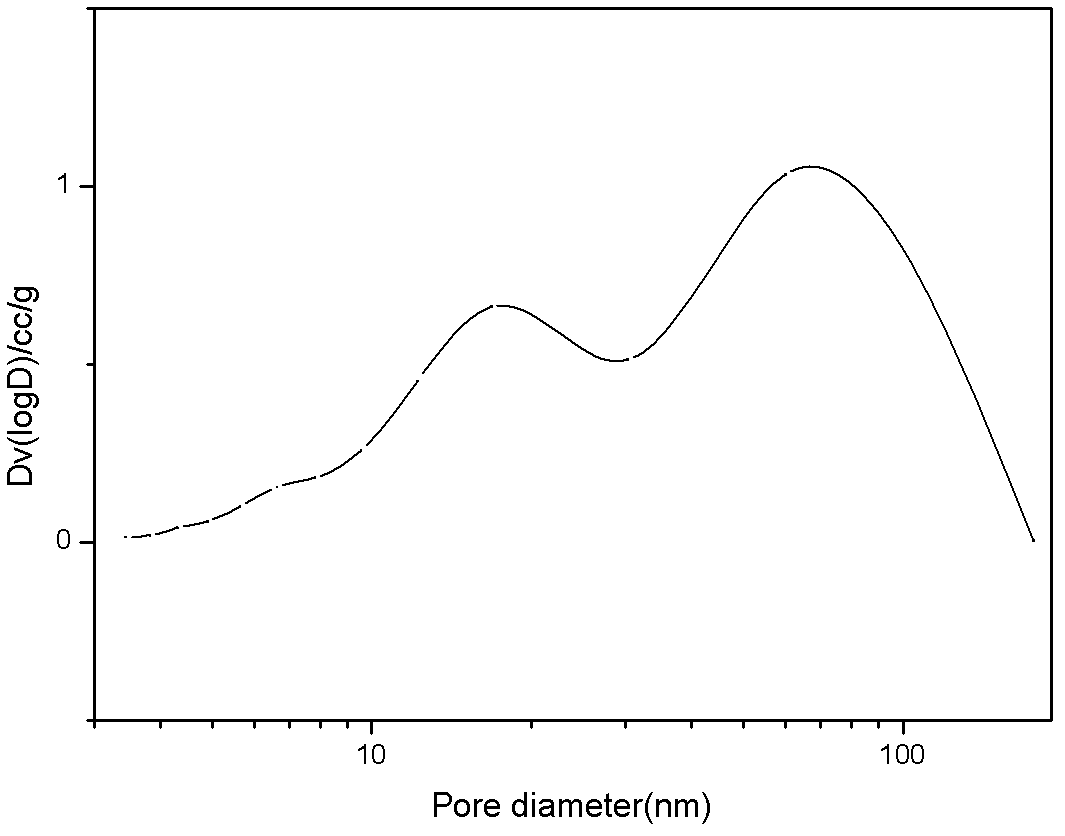
Double-hole carrier iron/ copper low-carbon alcohol synthesis catalyst and preparation method thereof
ActiveCN102631927AWide variety of sourcesLow costOrganic compound preparationHydroxy compound preparationColloidal silicaAlcohol
The invention provides a double-hole carrier iron / copper low-carbon alcohol synthesis catalyst with high activity and C<2+> alcohol selectivity, and a preparation method of the double-hole carrier iron / copper low-carbon alcohol synthesis catalyst. The double-hole carrier iron / copper low-carbon alcohol synthesis catalyst comprises Cu, Fe, K and M by weight percentage: 10-40% of Cu, 1-10% of Fe, 0.5-10% of K and 40-80% of M, wherein M is a double-hole carrier prepared from macroporous silica gel and ostiole colloidal silica. The preparation method comprises the steps of: dipping the ostiole colloidal silica in the macroporous silica gel in an isopyknic way, and preparing the double-hole carrier; then, dissolving metal in distilled water in a form of nitrate, and forming a mixed solution; and dipping the double-hole carrier in the mixed solution in an isopyknic way, and preparing the catalyst. The double-hole carrier iron / copper low-carbon alcohol synthesis catalyst prepared by the invention can be applied to the reaction for preparing low-carbon alcohol by synthesis gas, is simple in preparation technology, easy to operate, high in strength of the prepared catalyst and good in stability, and has high activity and C<2+> alcohol selectivity.
Owner:GUANGZHOU INST OF ENERGY CONVERSION - CHINESE ACAD OF SCI
Process for preparing alpha-damascone
ActiveUS20180244613A1High yieldMild reaction conditionsOrganic compound preparationCarbonyl compound preparationNitroxyl radicalsKetone
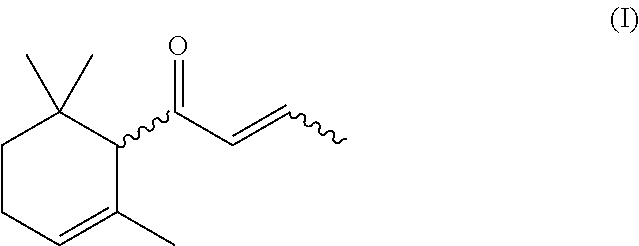
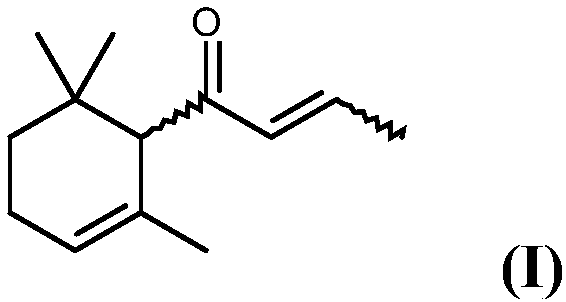
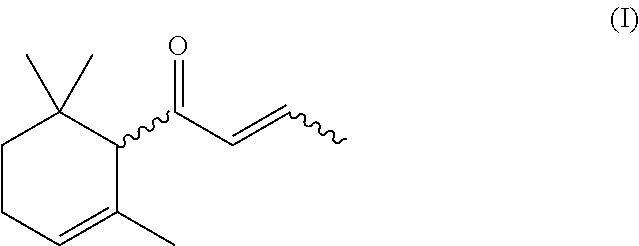
The present invention relates to a process for preparing 1-(2,6,6-trimethylcyclohex-2-en-1-yl)but-2-en-1-one, which comprises a) providing 6,10-dimethylundeca-1,5,9-trien-4-ol, b) oxidizing 6,10-dimethylundeca-1,5,9-trien-4-ol provided in step a) with an oxidizing agent in the presence of at least one organic nitroxyl radical, at least one nitrate compound and an inorganic solid to yield 6,10-dimethylundeca-1,5,9-trien-4-one, c) reacting the 6,10-dimethylundeca-1,5,9-trien-4-one obtained in step b) with an acid to yield 1-(2,6,6-trimethylcyclohex-2-en-1-yl)but-2-en-1-one.
Owner:BASF AG
Preparation method of catalyst with modified binderless ZSM-11 molecular sieves and application
ActiveCN107398294AImprove conversion rateMolecular sieve catalystsMolecular sieve catalystMolecular sieveAlkyl transfer
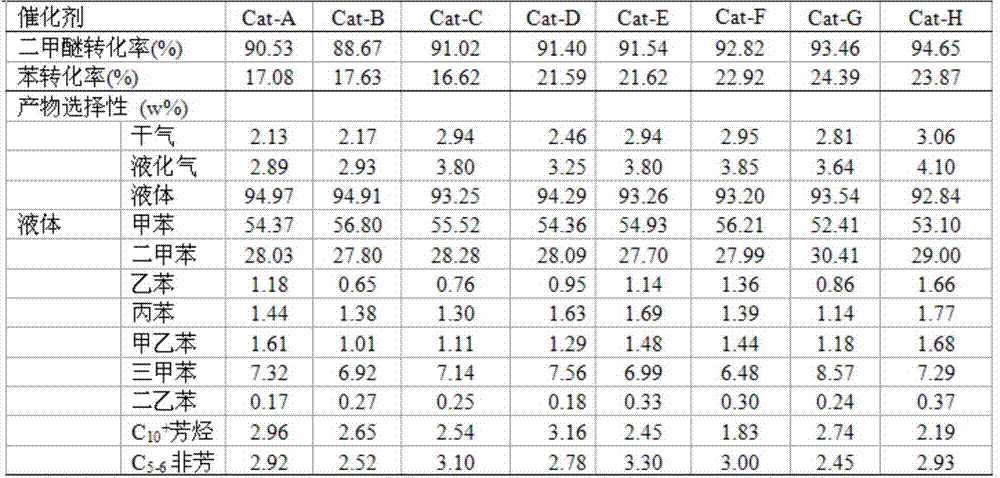
The invention discloses a preparation method of a catalyst with modified binderless ZSM-11 molecular sieves and an application. The preparation method comprises the specific steps: exchanging the prepared binderless ZSM-11 molecular sieves with an ammonium nitrate solution, drying and roasting to prepare an H-molecular sieve catalyst; and adopting nitrates and aluminium salts of rare earth to load rare earth and aluminium on the H-molecular sieve catalyst by a conventional impregnation method, and carrying out steam treatment to prepare the needed catalyst. When the catalyst prepared by the invention is applied to alkylation reaction of dimethyl ether and benzene, the aromatics with high yield can be generated. The preparation method disclosed by the invention has the advantages that compared with the catalyst only containing the binderless ZSM-11 molecular sieves, the benzene conversion ratio can be obviously increased.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Process for preparing alpha-damascone
PendingCN107922300ALess quantityProcess suitability is simple and inexpensiveOrganic compound preparationCarbonyl compound preparationNitroxyl radicalsAlpha-damascone
Owner:BASF SE
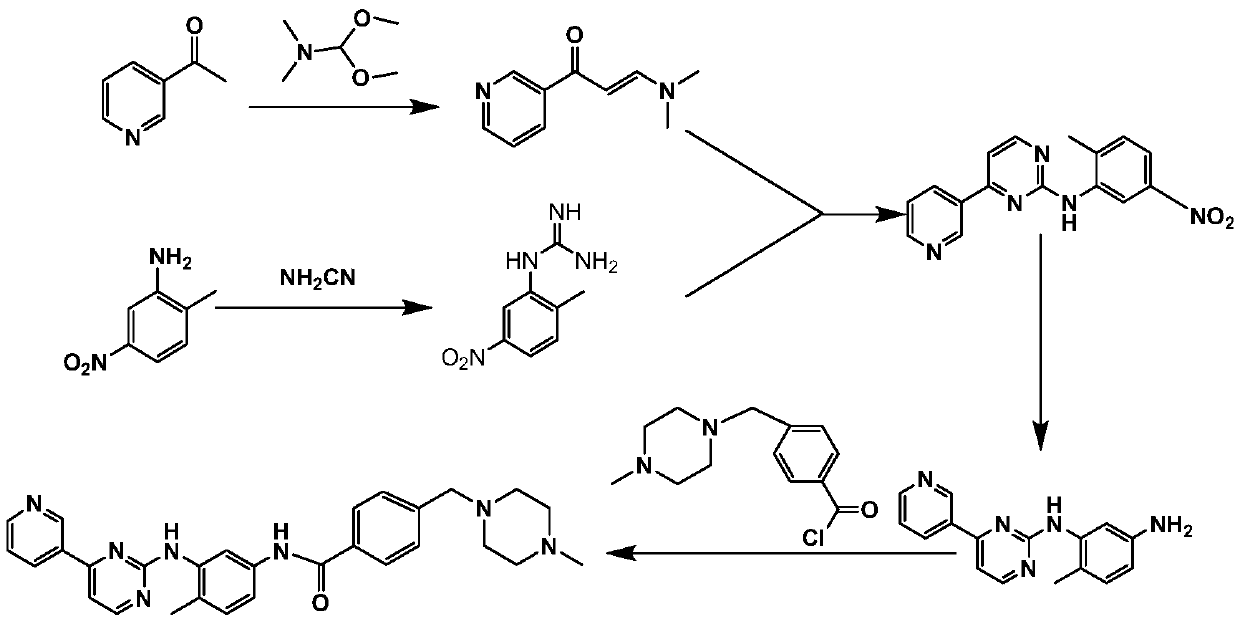
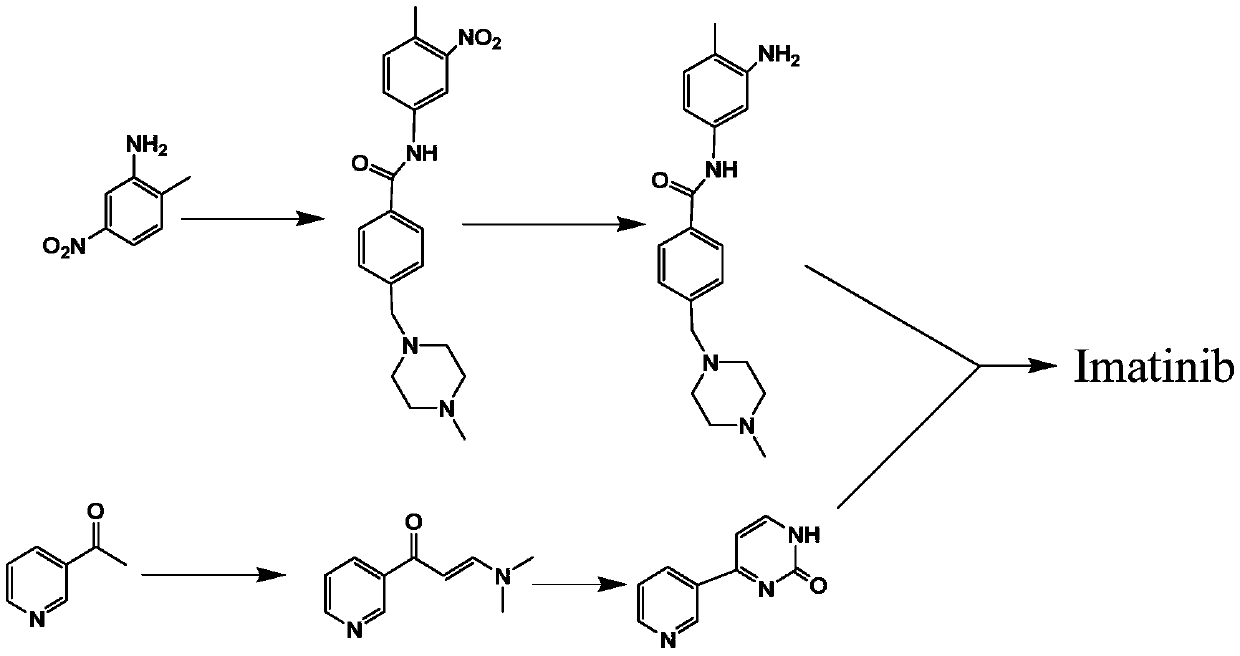
Synthesis method of imatinib and imatinib mesylate
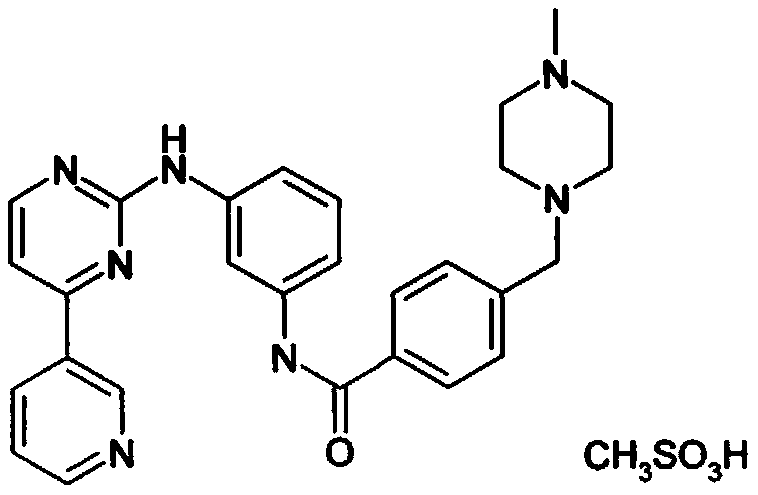
PendingCN111039921AOverall Reaction Time ReducedEasy to operateSulfonic acids salts preparationDimethyl acetalNitro reduction
The invention relates to a synthesis method of imatinib and imatinib mesylate. The method comprises the following steps: condensing 3-acetylpyridine and N,N-dimethylformamide dimethyl acetal which aretaken as initial raw materials to obtain 3-dimethylamino-1-(3-pyridyl)-2-propen-1-one, then reacting with 2-methyl-5-nitrophenylguanidine nitrate to form a pyrimidine ring, performing nitro reductionto obtain N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine, amidating the N-(5-amino-2-methylphenyl)-4-(3-pyridyl)-2-pyrimidinamine and 4-(chloromethyl)benzoyl chloride, performing affinitysubstitution with 1-methylpiperazine to obtain imatinib, and salifying the imatinib and methanesulfonic acid. The products obtained by the method have the advantages of few impurities, simplicity in post-treatment, high total yield, greenness, environmental protection and safety, and is suitable for a production process for large-scale industrial production of imatinib mesylate.
Owner:杭州沧海帆医药科技有限公司
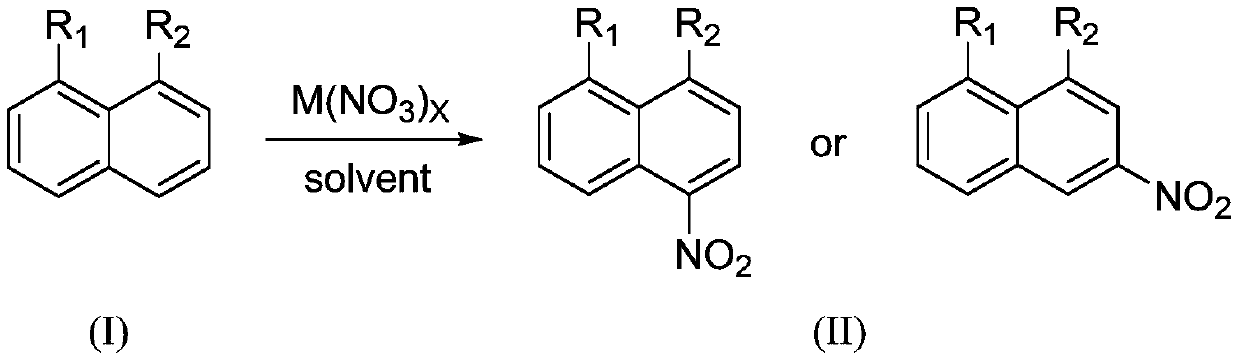
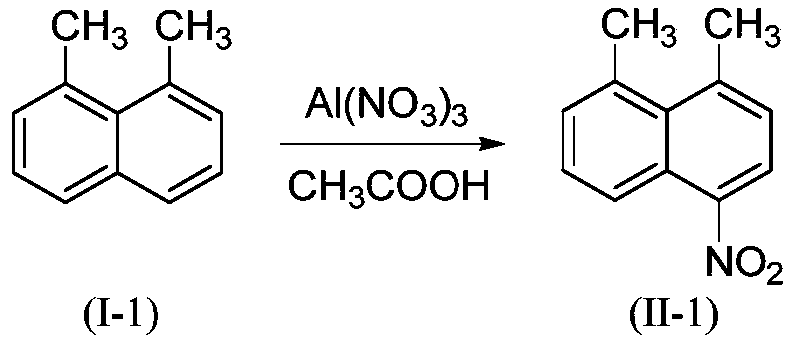
Preparation method of 1,8-disubstituted naphthalene mononitration derivative
ActiveCN110041202AHigh yieldHigh purityOrganic compound preparationAmino compound preparationMetal nitrateOrganic solvent
The invention provides a preparation method of 1,8-disubstituted naphthalene mononitration derivative. The preparation method is characterized by comprising the following steps: S1, taking 1,8-disubstituted naphthalene as a raw material, taking main group metal nitrate as a nitration reagent, dissolving the 1,8-disubstituted naphthalene and the main group metal nitrate in an organic solvent, performing a nitration reaction under 10-60 DEG C for 4-10 h, and then monitoring by TLC (Thin layer chromatography) till a raw material point disappears to finish the reaction; S2, cooling a product obtained in step S1 to room temperature, performing suction filtration, washing filter cake with 5-10 mL of H2O and anhydrous C2H5OH separately, and performing vacuum drying to obtain the 1,8-disubstitutednaphthalene mononitration derivative. According to the preparation method of the 1,8-disubstituted naphthalene mononitration derivative, the product yield can reach 90%-95%; the product purity reaches 98.5%-99.6%; compared with the prior art, the preparation method has the characteristics of high product yield, high purity, low cost and simple process, and is easy to industrialize.
Owner:YANGTZE UNIVERSITY
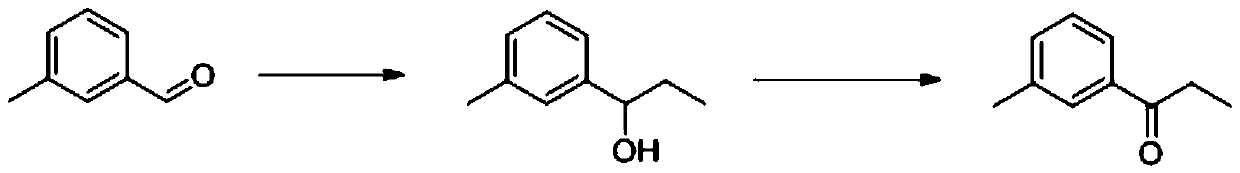
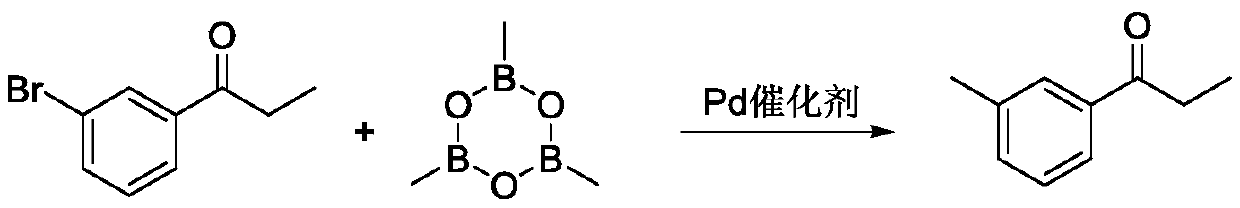
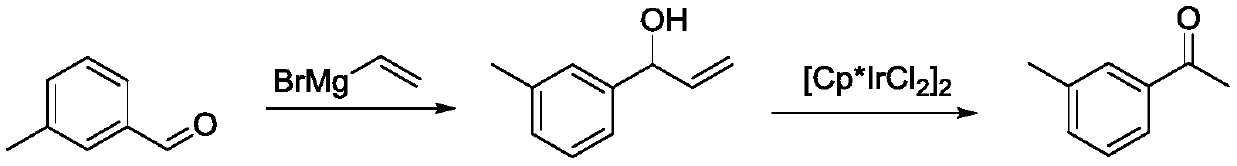
Synthetic method of 3 '-methyl propiophenone
PendingCN111393272AGood choiceHigh yieldOrganic compound preparationHydroxy compound preparationPtru catalystOrganic synthesis
The invention belongs to the field of organic synthesis, and discloses a synthetic method of 3 '-methyl propiophenone. The method comprises the following steps: taking m-tolualdehyde as a raw material, carrying out affinity addition reaction with an ethyl Grignard reagent to obtain 3 '-methyl phenylpropanol; and then using oxygen as an oxidizing agent, using a composite catalyst composed of nitroxide free radicals, inorganic bromide and nitrite, and performing catalytic oxidation on 3 '-methyl phenylpropanol to generate 3'-methyl propiophenone. The adopted composite catalyst is good in stability and selectivity, high in yield (up to 90% or above), reusable, safe and environment-friendly in the whole process and suitable for industrial production.
Owner:惠泽化学科技(濮阳)有限公司 +1
A kind of preparation method of fluorescent probe
ActiveCN108530459BRealize quantitative detectionAchieving selective recognitionOrganic chemistryFluorescence/phosphorescenceFluoProbesNitrite
The invention relates to a fluorescent probe preparation method. Specifically, the probe disclosed by the invention is benzothiazole-rhodamine compound and can serve as a nitrite peroxide ratio fluorescent probe to be applied to nitrite peroxide detection. The preparation method disclosed by the invention utilizes a one-step synthesizing method, so that the preparation method has the advantages ofsimpleness in preparation and high yield.
Owner:杭州佰迈贝生物科技有限公司
Mefenpyr-diethyl impurity, preparation method and applications thereof
InactiveCN110724144AHigh economic feasibilityMild reaction conditionsOrganic chemistry methodsTesting foodCarboxylic acidStructural formula
The invention discloses a mefenpyr-diethyl impurity, a preparation method and applications thereof, wherein the molecular structural formula is represented by a formula I. The preparation method comprises: carrying out diazotization and a Japp-Klingemann reaction by using 2,4,-dichloroaniline, nitrite and ethyl 2-chloroacetoacetate as raw materials to obtain an intermediate, and carrying out a ring-closure reaction on the intermediate and ethyl methacrylate to obtain 1,5-bis(2,4-dichlorophenyl)-6-methyl-1H,5H-pyrazolo[5,1-c]-1,2,4-triazole-3,6,7a-triethyl tricarboxylate, wherein the yield reaches more than 40%. According to the invention, the method has advantages of mild reaction conditions, low energy consumption, high economic feasibility and high yield; and the substance has the application value in the influence on the efficacy of mefenpyr-diethyl by using the substance as the impurity and in the safety evaluation of the residual substance on agricultural products.
Owner:江苏艾科姆检测有限公司
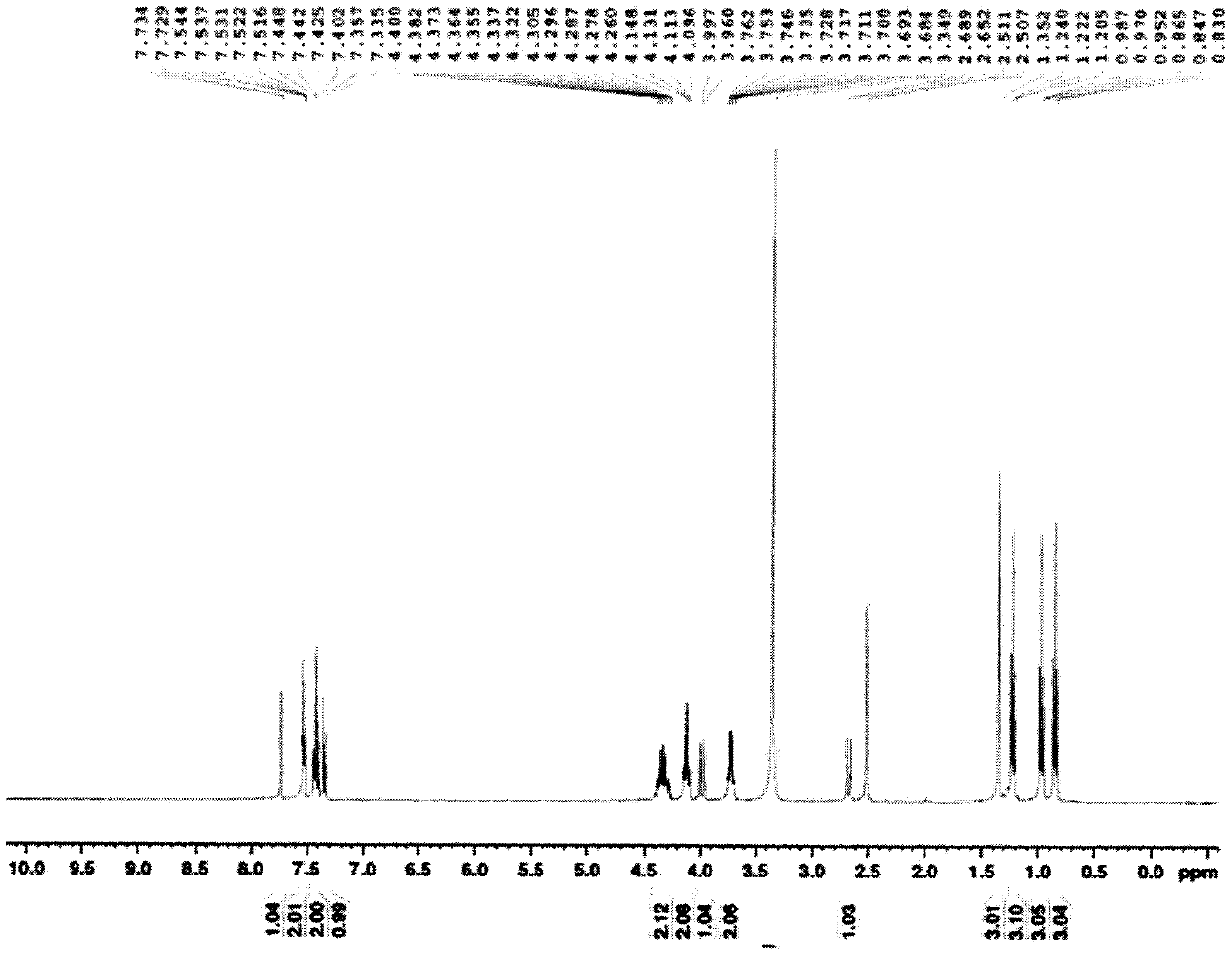
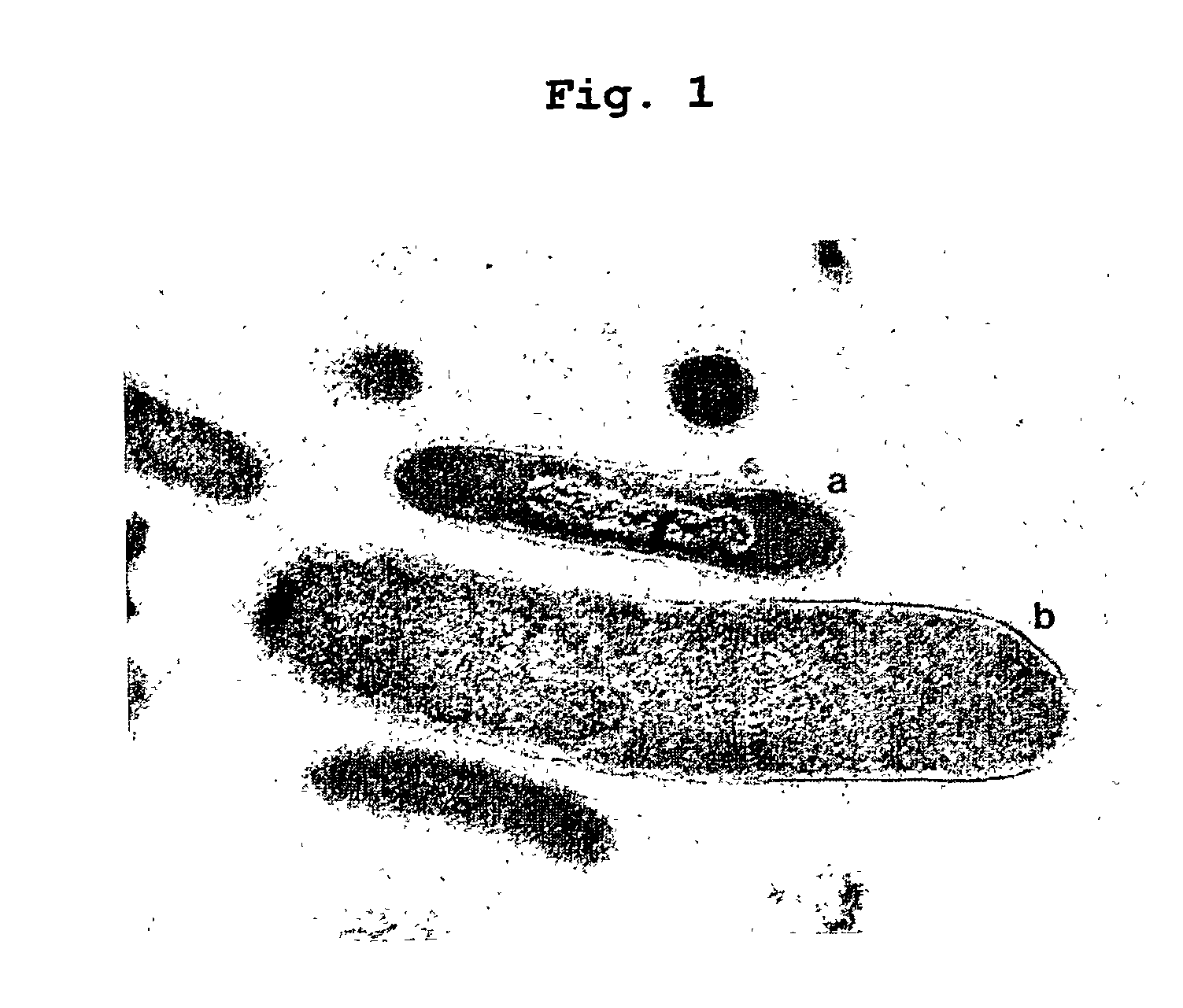
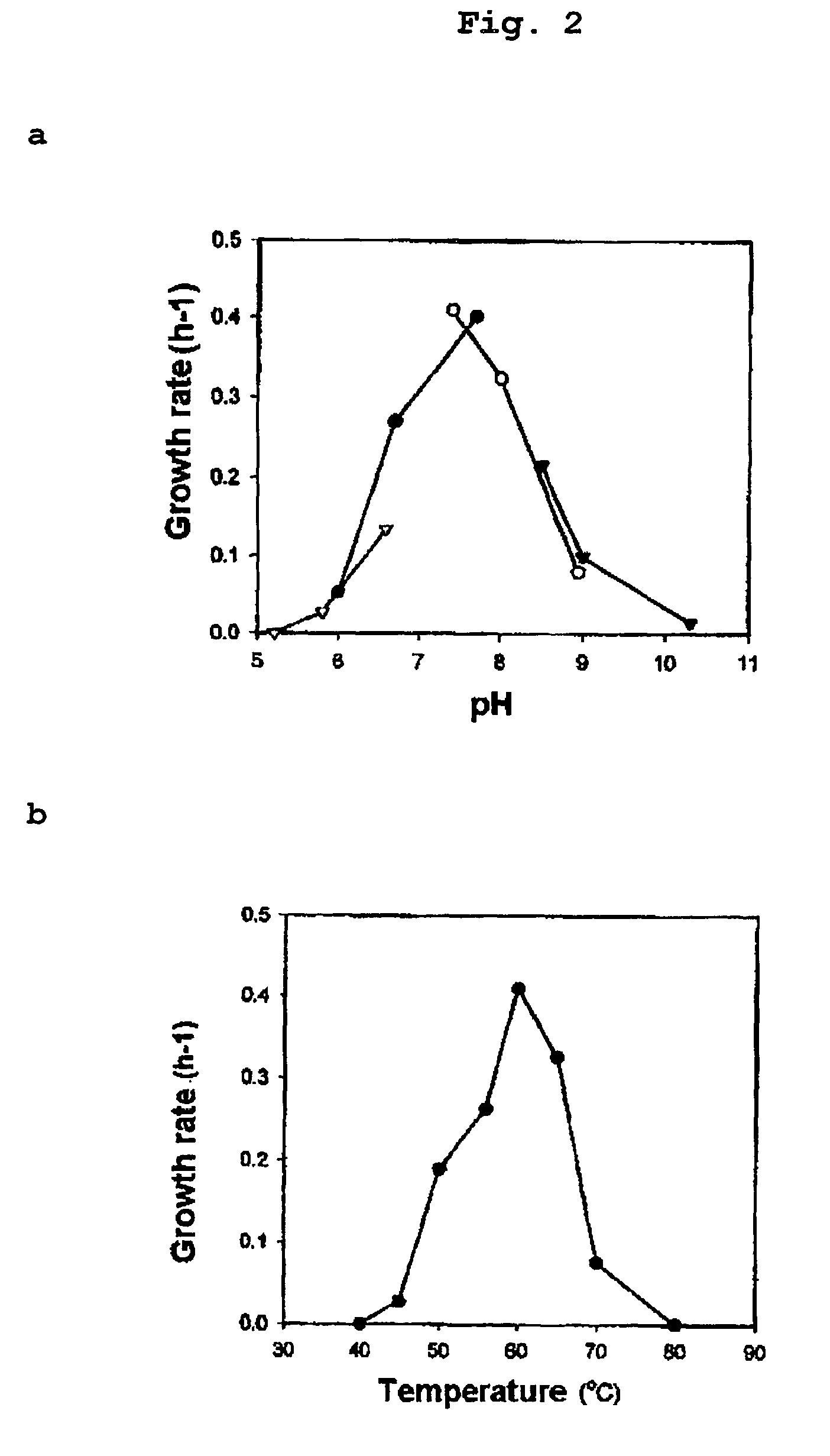
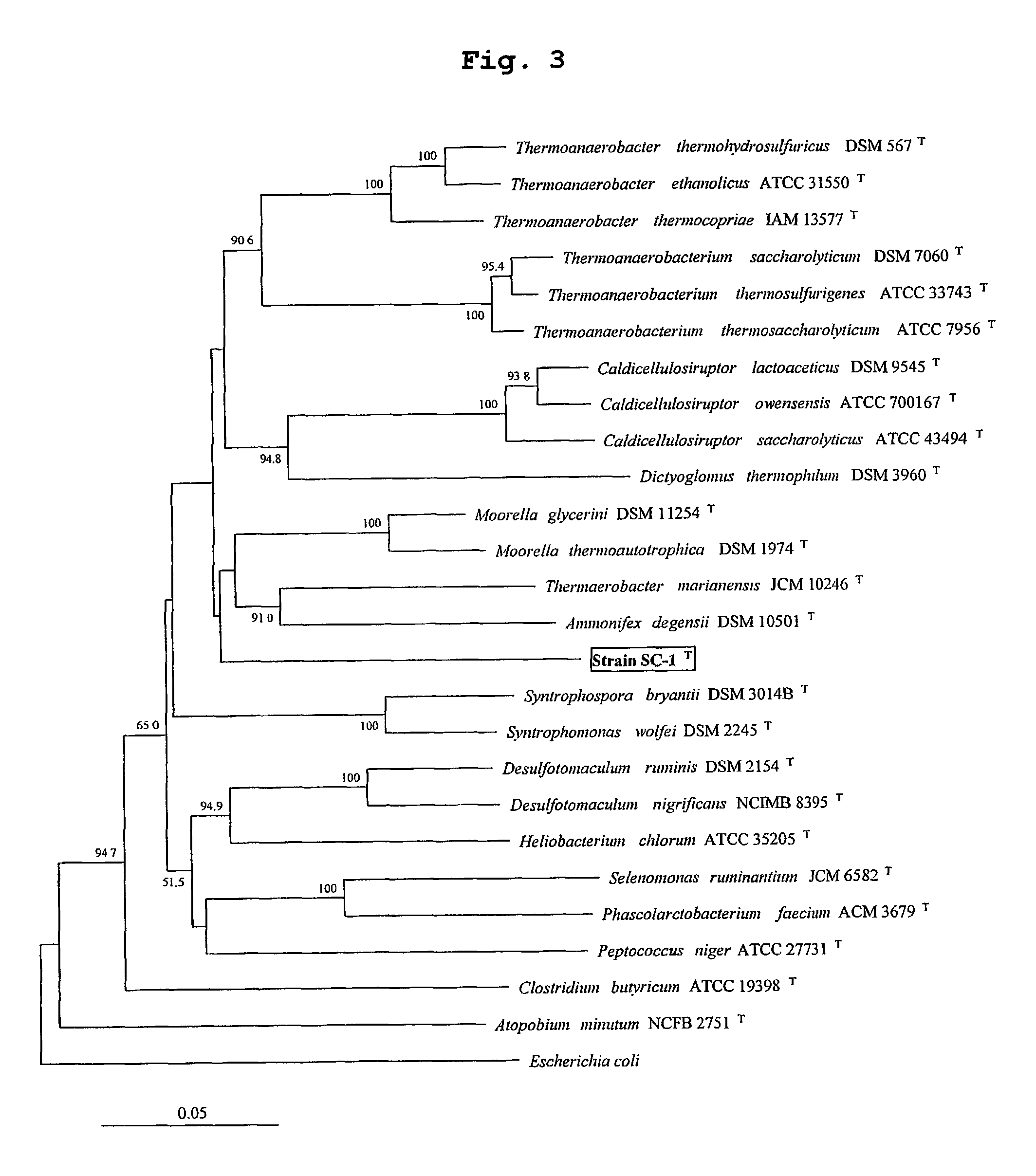
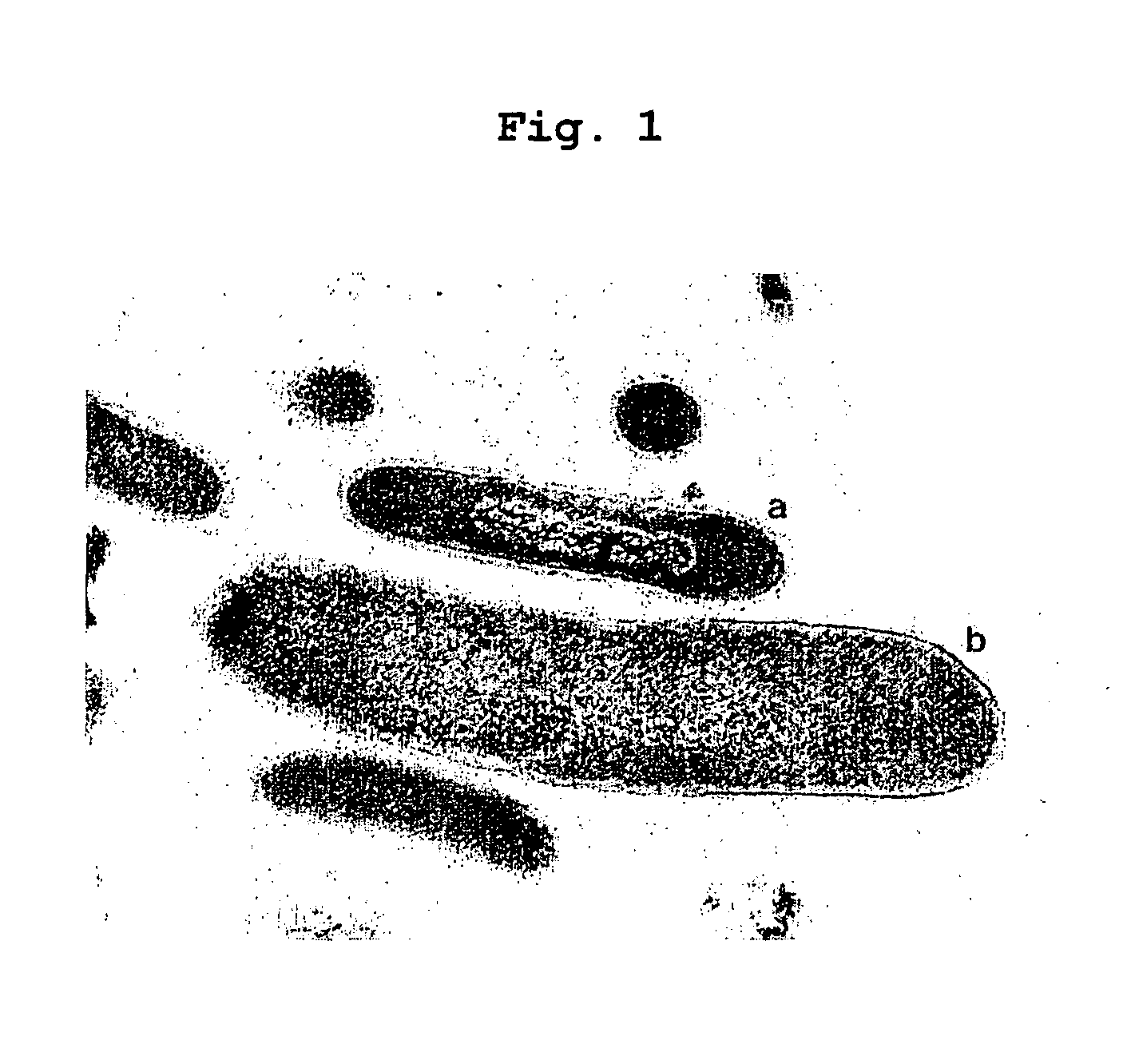
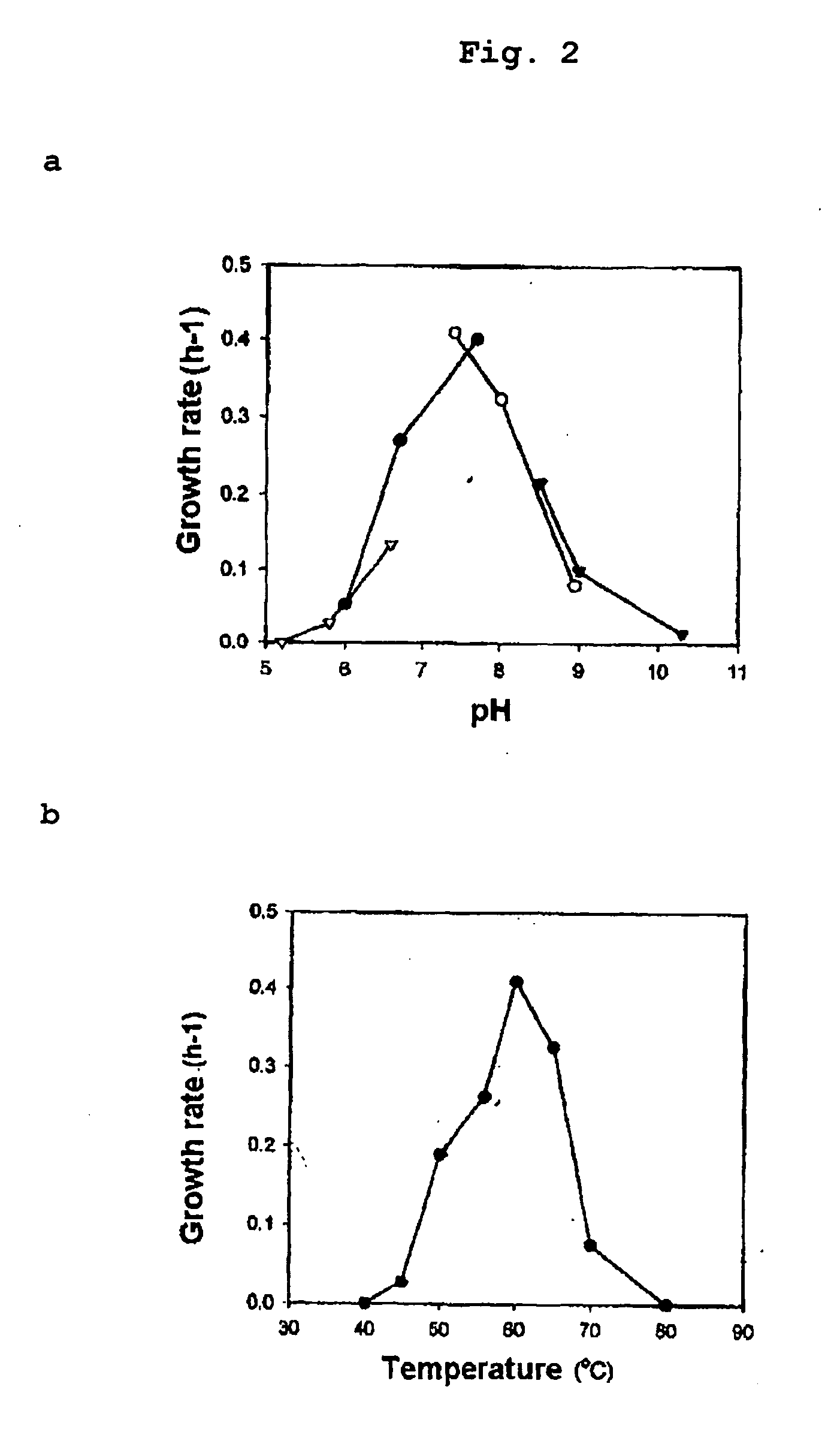
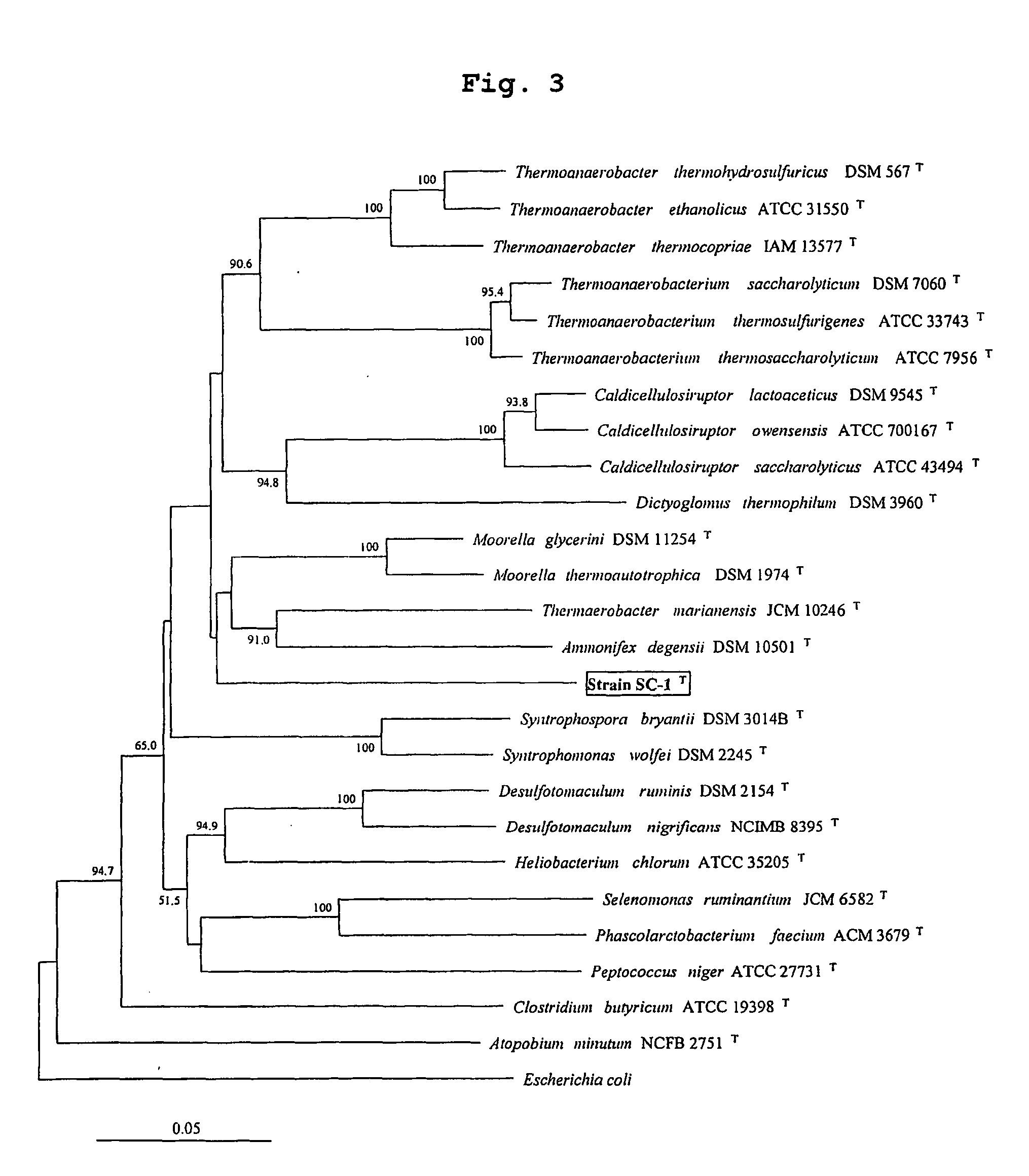
Obligately symbiotic thermophile symbiobacterium toebii SC-1 producing thermostable L-tyrosine phenol-lyase and L-tryptophan indole-lyase and a method for screening its relative bacteria
InactiveUS6967087B1BacteriaMaterial analysis by observing effect on chemical indicatorBacteroidesSymbiotic bacteria
The present invention relates to a novel obligately symbiotic thermophile Symbiobacterium toebii SC-1(Accession NO: KCTC 0685BP), and a thermostable L-tyrosine phenol-lyase and L-tryptophan indole-lyase produced by Symbiobacterium toebii SC-1. The present invention also relates to a pure culture method of Symbiobacterium toebii SC-1, a method of growth measurement for Symbiobacterium toebu SC-1 and its relative symbiotic bacteria showing low-growth yield using nitrate respiration, and a screening method of relative symbiotic strains of Symbiobacterium toebii SC-1 from the environment using a specific antibody to it. Therefore, the thermostable L-tyrosine phenol-lyase and L-tryptophan indole-lyase produced by Symbiobacterium toebii SC-1 in this invention can provide more stable catalysts in enzymatic biotransformation processes which enzymatically produce valuable medico-amino acids 3,4-dehydroxy-L-phenylalanine (L-DOPA) and L-tryptophan.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
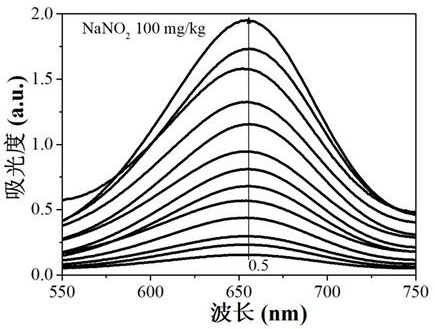
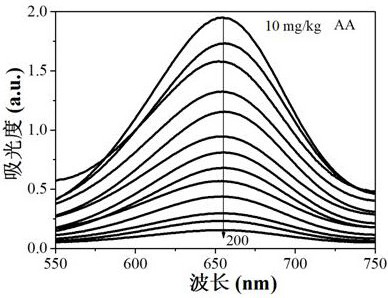
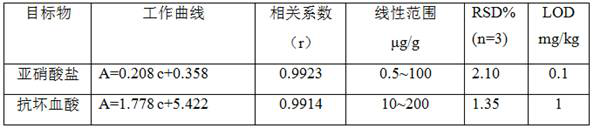
Method for simultaneously detecting nitrite and ascorbic acid in food
PendingCN112326579ASensitive highHigh selectivityColor/spectral properties measurementsBiotechnologyOctanoic Acids
The invention discloses a method for simultaneously detecting nitrite and ascorbic acid in food. According to the method, colorless 3, 3', 5, 5'-tetramethyl benzidine (TMB) is oxidized into a blue solution in the presence of hydrogen peroxide through the pseudo peroxidase characteristic of an octanoic acid coated ferroferric oxide nano material; the nitriteaccelerates the oxidation reaction, and the ascorbic acid can fade blue, but ascorbic acid in the sample interferes with determination of the nitrite, and interference is eliminated through treatment of modified activated carbon; based on the linear relationship between the nitrite concentration and the absorbance increase and the linear relationship between the ascorbic acid concentration and the absorbance decrease, a novel high-sensitivity and high-selectivity nitrite and ascorbic acid detection method is established, and the detection limits are 0.1 mg / kg and 1mg / kg respectively. The method is applied to detection and analysis ofnitrite and ascorbic acid in food, and results of the method accord with those of related national standard determination methods. The method has the advantages of simple operation, high sensitivity,rapidness and the like.
Owner:AGRO PROD PROCESSING RES INST YAAS
Organic amine dimmer and method for synthesizing the same
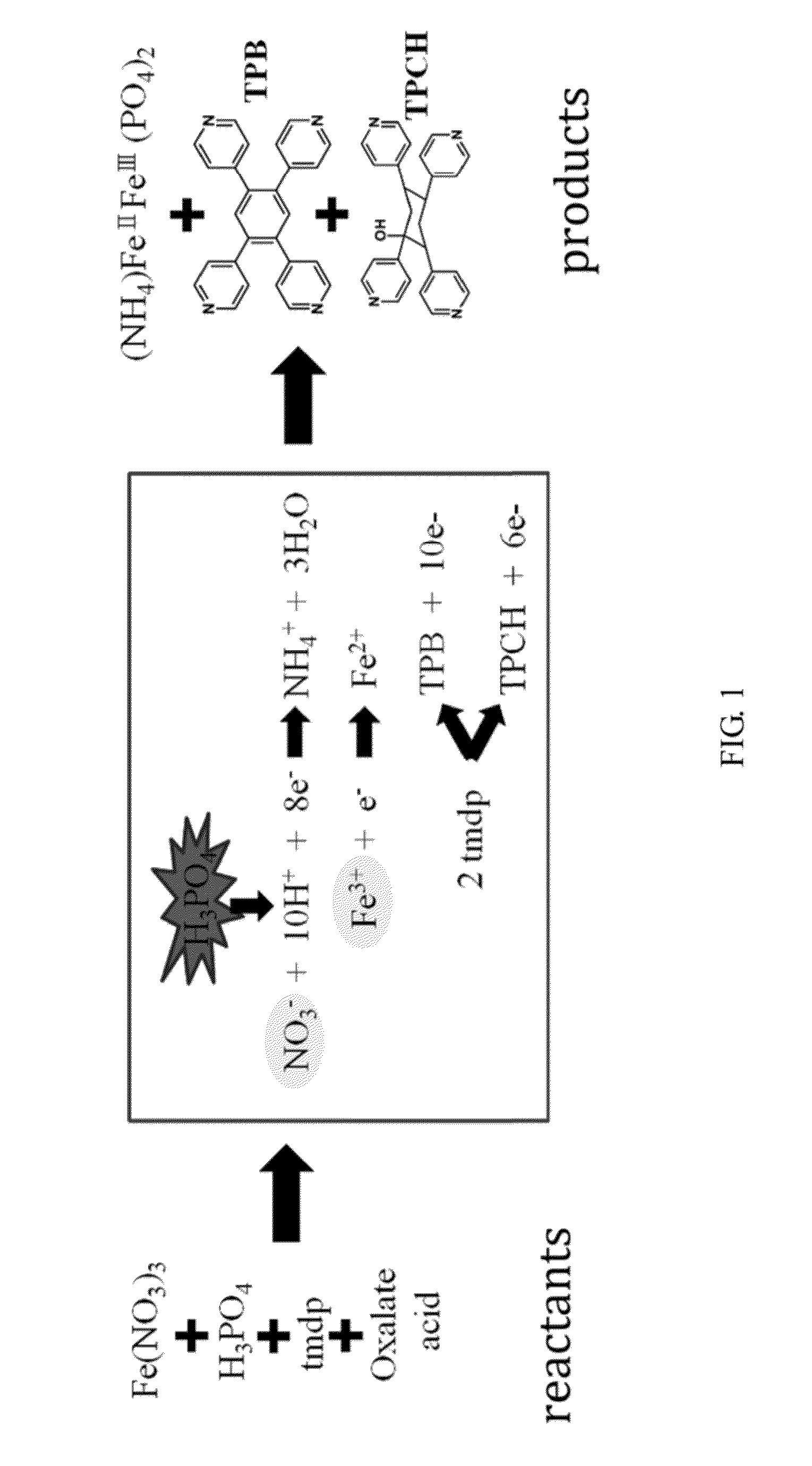
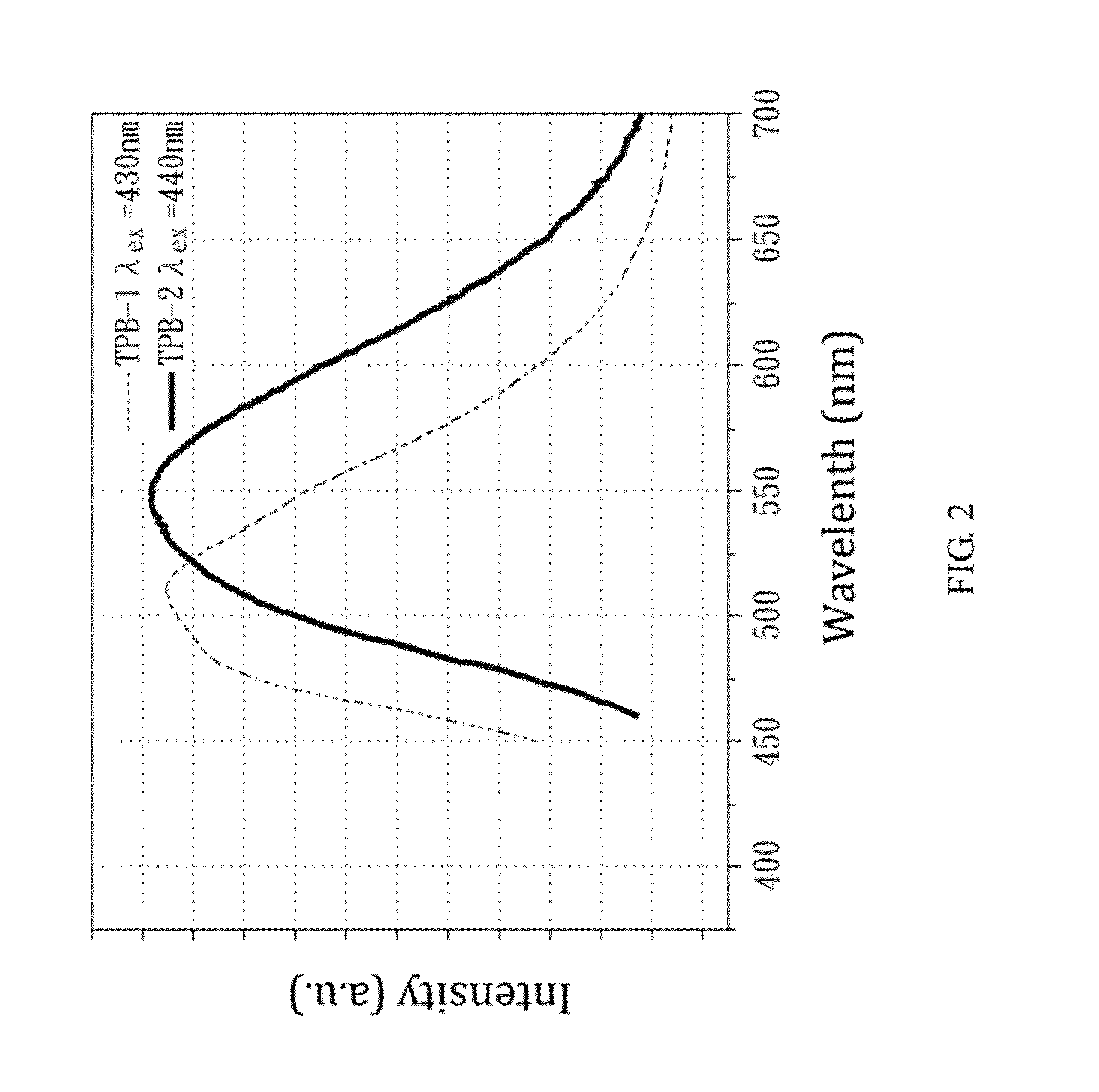
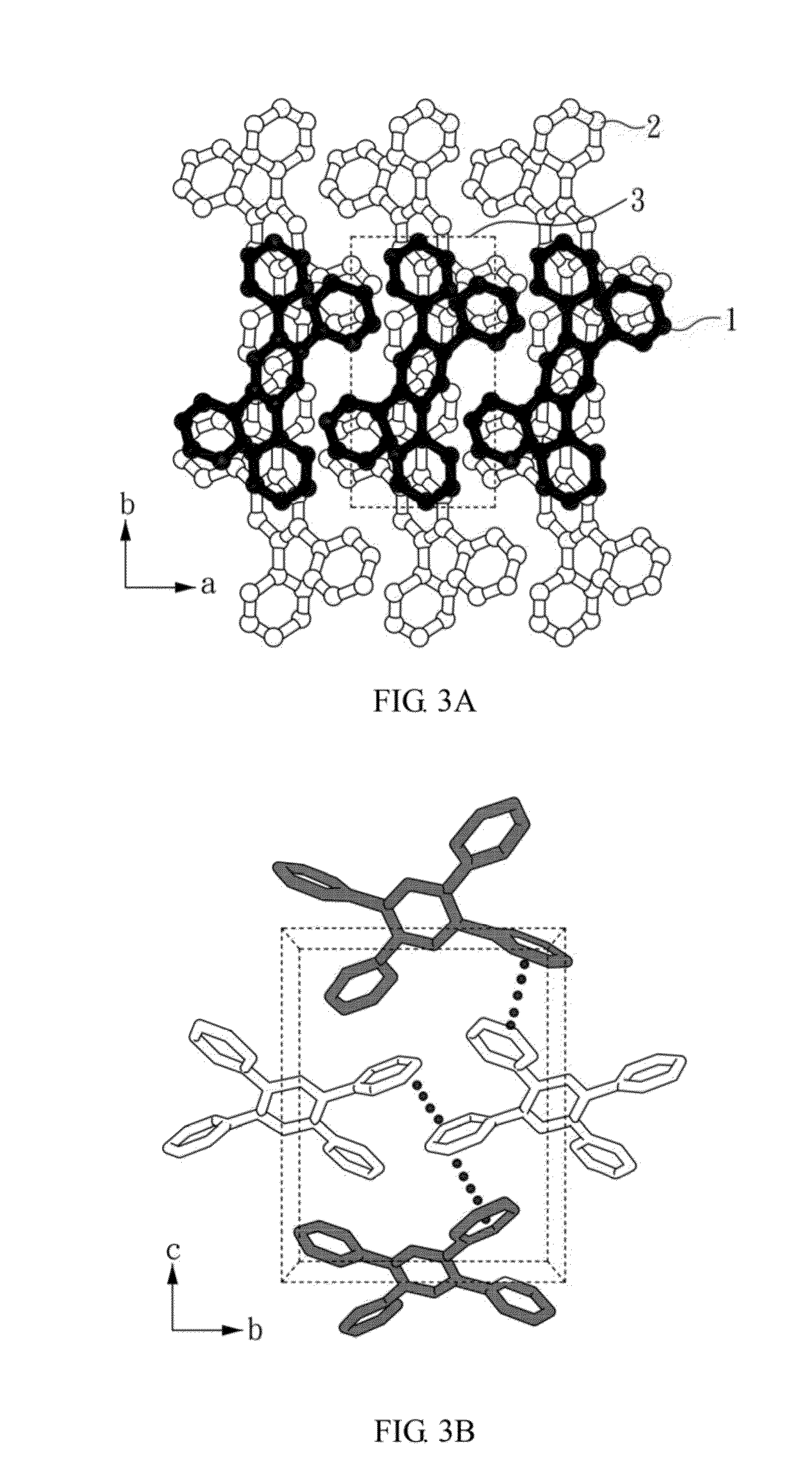
An organic amine dimmer and a method for synthesizing the same are disclosed. The method comprises the following steps: (A) mixing an iron-containing compound (e.g., Fe2O3, FeCl3), a nitrate-containing compound, oxalate acid, 4,4′-trimethylenedipiperidine (TMDP), H3PO4, and 2 to 8 ml of water to form a mixture; (B) heating the mixture and maintaining the mixture at 160° C. or above for a first predetermined time; (C) cooling the mixture; and (D) placing the mixture at room temperature for a second predetermined time, to obtain an organic amine dimmer, wherein Fe(NO3)3 can serve as the iron-containing compound as well as the nitrate-containing compound.
Owner:NATIONAL TSING HUA UNIVERSITY
Process for preparing mono-long chain amine oxide surfactants with low nitrite, nitrosamine and low residual peroxide level
InactiveCN1328543AOrganic chemistrySurface-active detergent compositionsActive agentSurface-active agents
Mono-long chain amine oxide surfactants can be prepared containing active levels of greater than about 30%, by weight of amine, and with low nitrite and nitrosamine levels and low levels of residual hydrogen peroxide when oxidized under controlled temperature conditions in the presence of bicarbonate material present at levels less than about 2.5%, by weight of amine, and without the presence of phase-modifying solvents.
Owner:THE PROCTER & GAMBLE COMPANY
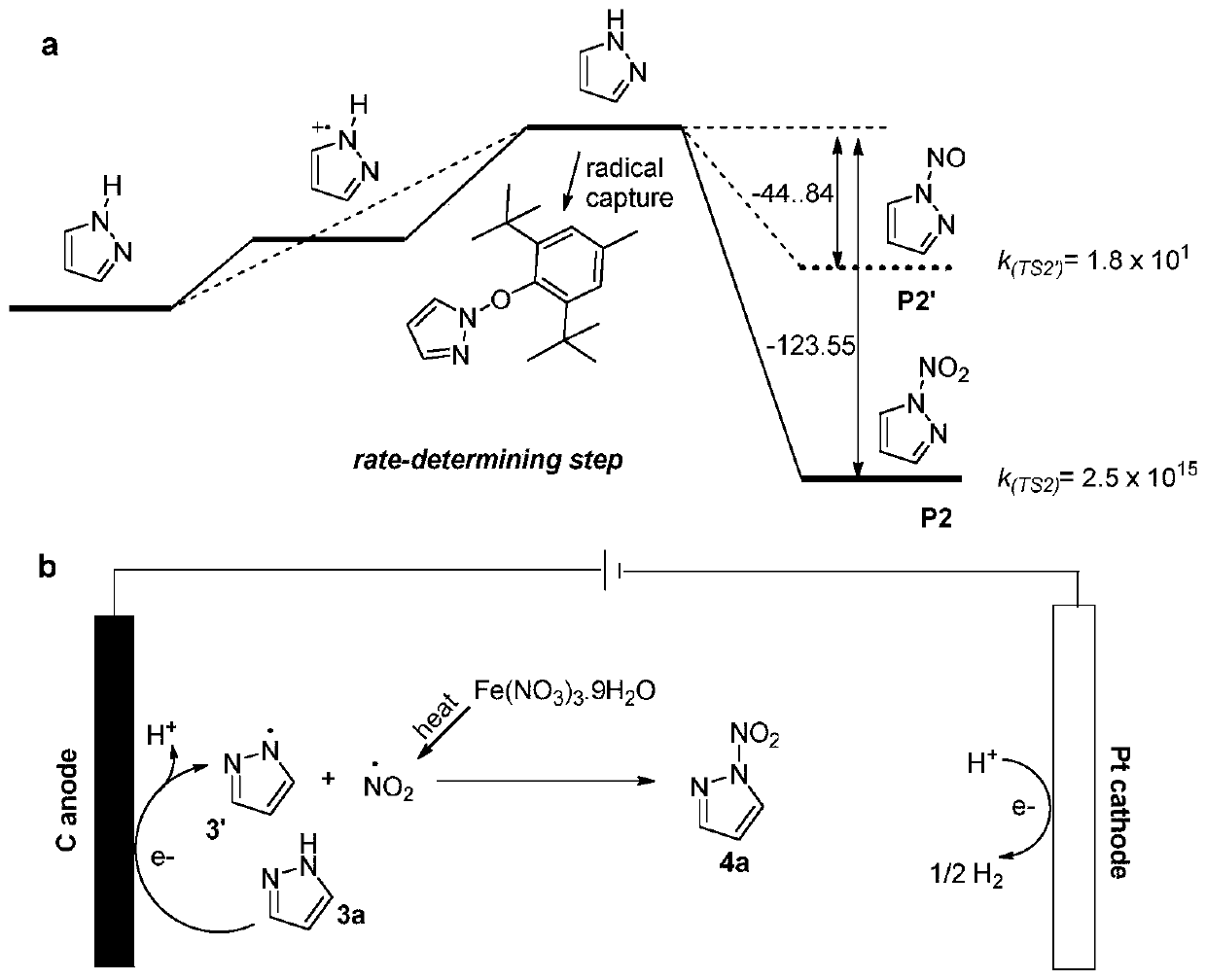
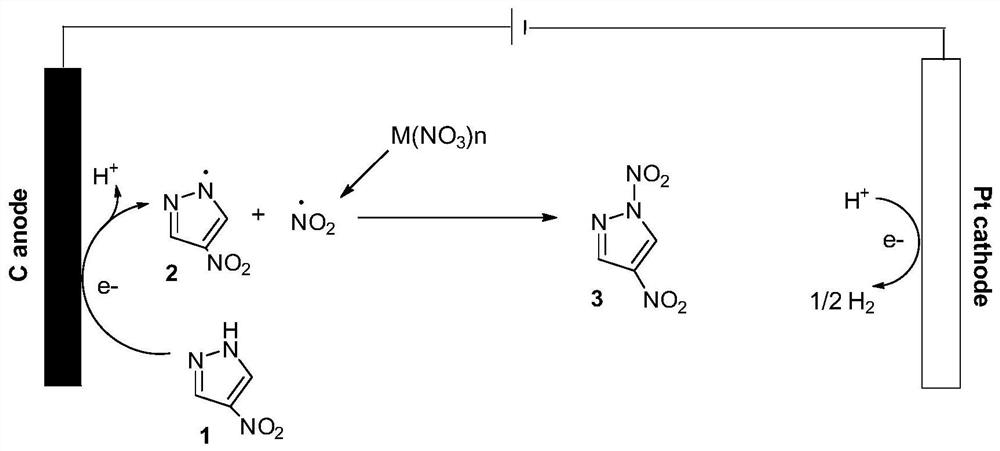
Synthesis method of nitroazole energetic compound
ActiveCN111235597AHigh yieldImprove responseElectrolysis componentsElectrolytic organic productionMetal nitrateCombinatorial chemistry
The invention discloses a synthesis method of an azole N-nitrated compound, and the method comprises the following steps: carrying out electrocatalytic free radical coupling reaction, and carrying outnon-oxidative nitration reaction on the N-H bond of the azole by using economic and nontoxic metal nitrate as a nitro source. According to the method, an electro-catalysis mode is adopted, various cheap metal nitrates are adopted as a nitrate source, a target product is synthesized through a one-step method, the reaction process is simple, the substrate universality is good, the yield of the generated N-nitroazole compound is high, and a green N-N single bond construction method is achieved.
Owner:NANJING UNIV OF SCI & TECH
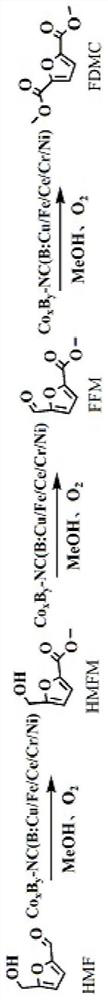
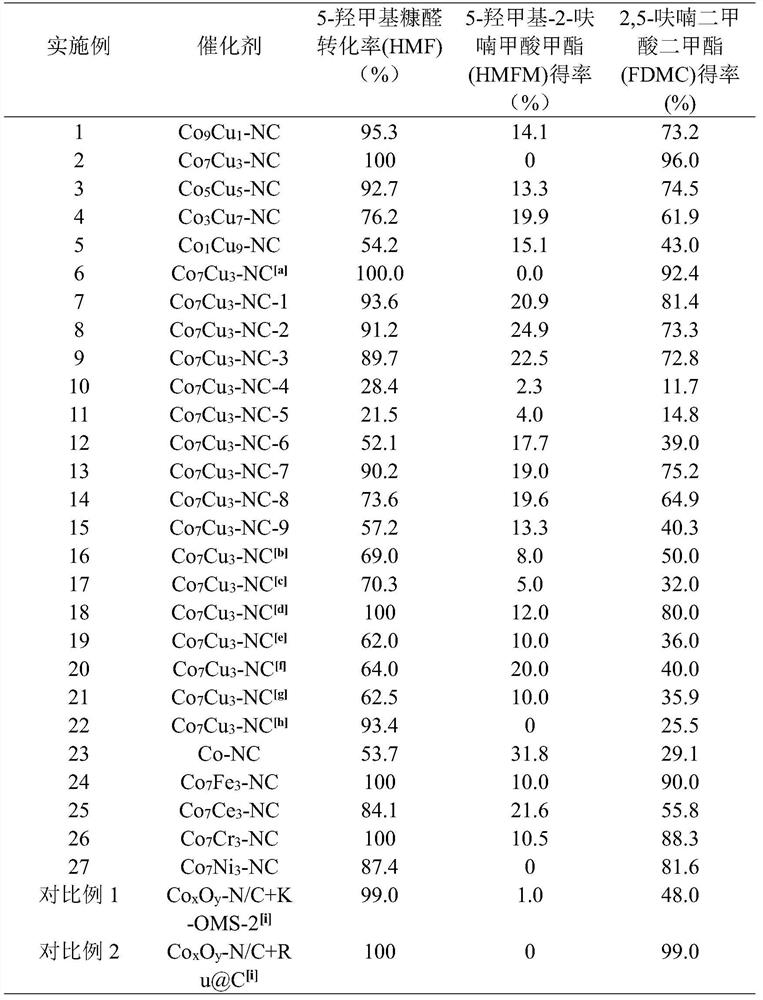
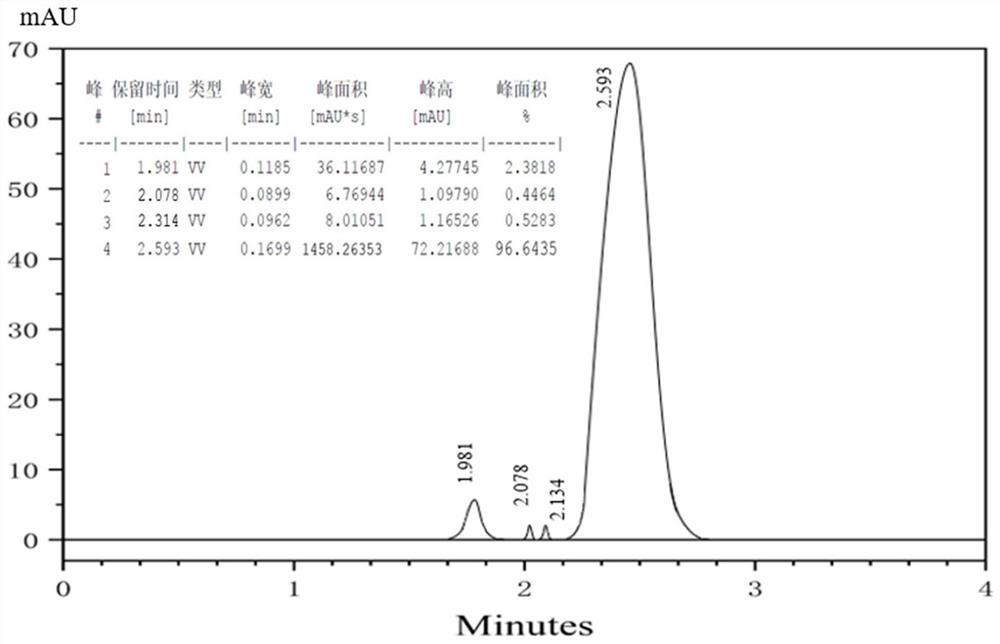
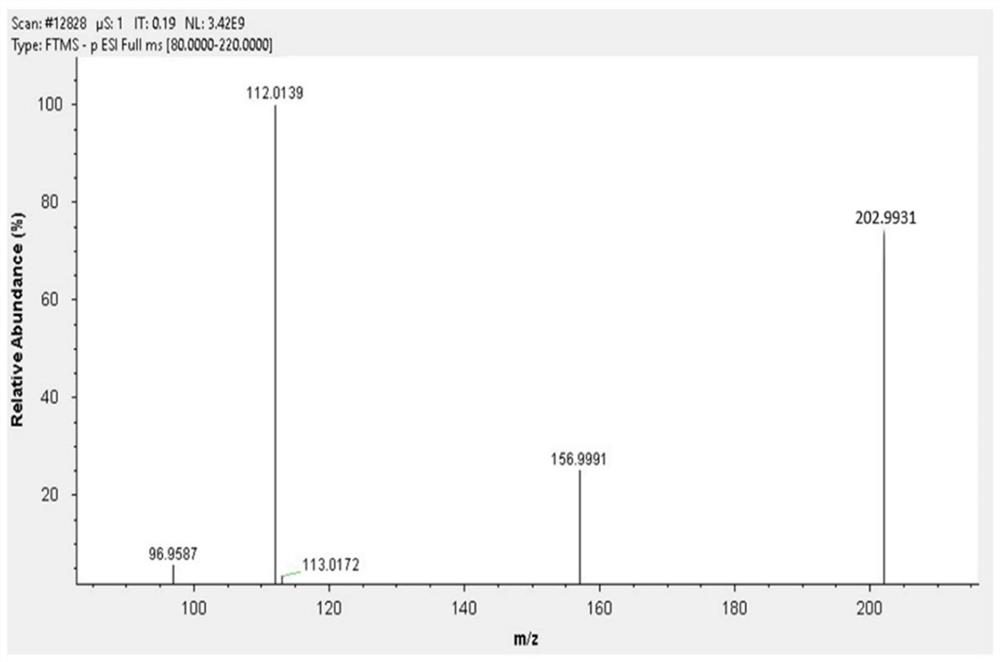
Nitrogen-carbon doped cobalt-based bimetallic catalyst, preparation method thereof and preparation method of 2, 5-dimethyl furandicarboxylate
ActiveCN113198512AEasy to prepareSuitable for industrial productionOrganic chemistryCatalyst activation/preparationFuranPtru catalyst
The invention provides a nitrogen-carbon doped cobalt-based bimetallic catalyst and a preparation method thereof. The preparation method comprises the following steps of mixing: mixing a nitrogen source compound, cobalt nitrate, a second metal element nitrate and a carbon source compound, stirring at 90-120 DEG C for more than 8 hours, filtering and drying to obtain a solid compound containing Co, a second metal element, N and C elements, and calcining: calcining the solid compound under a nitrogen condition to obtain the composite catalyst containing Co, a second metal element, N and C elements. The invention also provides a preparation method of 2, 5-dimethyl furandicarboxylate, which comprises the following steps: mixing 5-hydroxymethylfurfural, methanol and the nitrogen-carbon doped cobalt-based bimetallic catalyst, and reacting for 3-5 hours in an oxygen environment under the conditions of pressure of 0.1-0.5 MPa, temperature of 70-90 DEG C and stirring to obtain 2, 5-dimethyl furandicarboxylate. The catalyst is simple in preparation method and suitable for industrial production, and the yield of 2, 5-dimethyl furandicarboxylate can reach 96%. Under the condition that the concentration of HMF is 10 wt%, the yield of 2, 5-dimethyl furandicarboxylate is 92%.
Owner:莆田达凯新材料有限公司
Method for preparing 1,3,5-trinitropyrazole by electrochemical method
InactiveCN113737207AUniversalHigh yieldElectrolysis componentsElectrolytic organic productionMetal nitratePhysical chemistry
The invention discloses a method for preparing 1,3,5-trinitropyrazole through an electrochemical method, an N-nitropyrazole compound is prepared through electrochemistry, economical and nontoxic metal nitrate is used as a nitro source. According to the invention, a novel construction method of a trinitropyrazole energetic compound is realized through the reaction, by which a target product is obtained at a high yield under a constant current. The pyrazole ring structure has large ring tension and contains high-energy N-N bonds and C-N bonds. The nitro group is introduced on the ring, so that the density and the nitrogen content are increased, and the oxygen balance is closer to an ideal value, thereby improving the detonation performance of the compound.
Owner:NANJING UNIV OF SCI & TECH
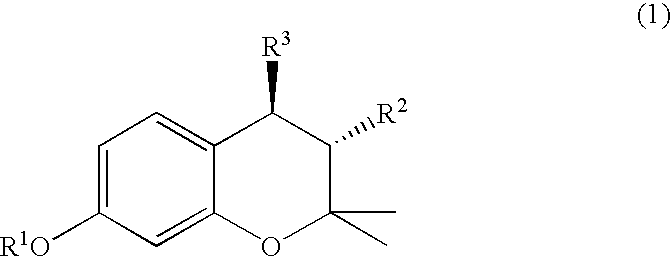
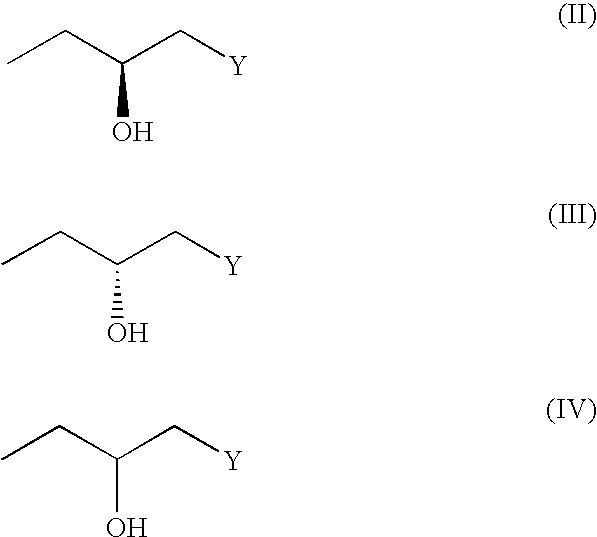
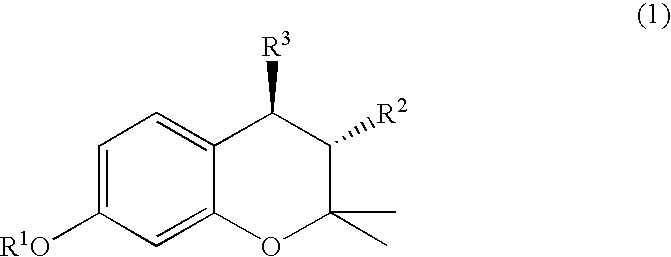
(3R, 4R)-trans-3, 4-diarylchroman derivatives and a method for the prevention and/or treatment of estrogen dependent diseases
The present invention relates to compounds of the formula I in which substituents R2 and R3 are arranged in trans-configuration:wherein:R1 is H or C1-C6 alkyl; C3-C7 cycloalkyl;R2 is phenyl, optionally substituted with 1 to 5 substituents independently selected from the group comprising OH, C1-C6-alkyl, halogen, nitro, cyano, SH, SR4, trihalo-C1-C6-alkyl, C1-C6-alkoxy and phenyl, wherein R4 is C1-C6 alkyl;R3 is phenyl substituted with OR5 wherein R5 has the formula (II), (III) or (IV)wherein Y is chosen from NHR4, NR42, NHCOR4, NHSO2R4, CONHR4, CONR4, CONR42, COOH, COOR4, SO2R4, SOR4, SONHR4, SONR42, a C3-C7 heterocyclic ring, saturated or unsaturated, containing one or two heteroatoms independently selected from the group consisting of O, S and N, optionally being substituted with 1 to 3 substituents independently selected from the group comprising H, OH, halogen, nitro, cyano, SH, SR4, trihalo-C1-C6-alkyl, C1-C6-alkyl and C1-C6-alkoxy, preferably NHR4, NR24, or a nitrogen heterocycle, wherein R4 is as defined above, and the esters, ethers, and salts of the compounds of formula I, optionally along pharmaceutically acceptable excipients, a process for the preparation of the same, and a method of preventing and / or treating estrogen-related disease conditions in a subject using compounds of formula 1, or its salts, optionally along with pharmaceutically acceptable excipients.
Owner:COUNCIL OF SCI & IND RES
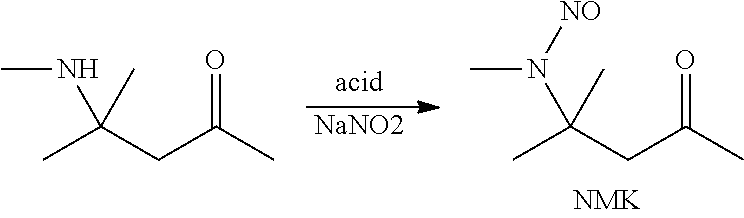
Process for the cyclopropanation of olefins using N-methyl-N-nitroso compounds
A process of converting a carbon-carbon double bond on a substrate into a cyclopropane ring, which method comprises the step of treating the substrate with a N-alkyl-N-nitroso compound, a transition metal catalyst and an aqueous base, wherein the N-alkyl-N-nitroso compound is formed by reacting an alkyl amine with an alkali metal nitrite in the presence of a mono-basic or di-basic acid, or a mixture thereof, and wherein the N-alkyl-N-nitroso compound is not distilled before it is mixed with the substrate, catalyst and base.
Owner:GIVAUDAN SA
Spiropyrane coordination compound photochromic material and preparation method thereof
PendingCN113045608ARealize photocatalytic degradationGroup 1/11 organic compounds without C-metal linkagesWater/sewage treatment by irradiationN-Propyl alcoholUltraviolet lights
The invention discloses a spiropyran coordination compound photochromic material, which is prepared from the following raw materials: nitrate, N-hydroxyethyl-3,3-dimethyl-6-nitro indoline spiropyran and aliphatic diamine. The preparation method comprises the following steps: (1) weighing raw materials; and (2) adding nitrate, N-hydroxyethyl-3,3-dimethyl-6-nitro indoline spiropyran and aliphatic diamine into N,N'-dimethylformamide and n-propyl alcohol, and standing at a constant temperature of 75-85 DEG C for 70-75 hours to obtain the product. According to the invention, a metal organic framework coordination compound is synthesized by using N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran as a ligand through a hydrothermal synthesis method, characterization results find that the spiropyran ligand is subjected to an in-situ reaction with ethanediamine and propane diamine to form a Schiff base ligand, the metals Ni and Cu are coordinated with the Schiff base ligand to form a tetra-coordinated coordination compound, and the photocatalytic degradation of dyes can be realized under the irradiation of ultraviolet light.
Owner:JIAXING UNIV +2
A kind of synthetic method of nitroalkene compound
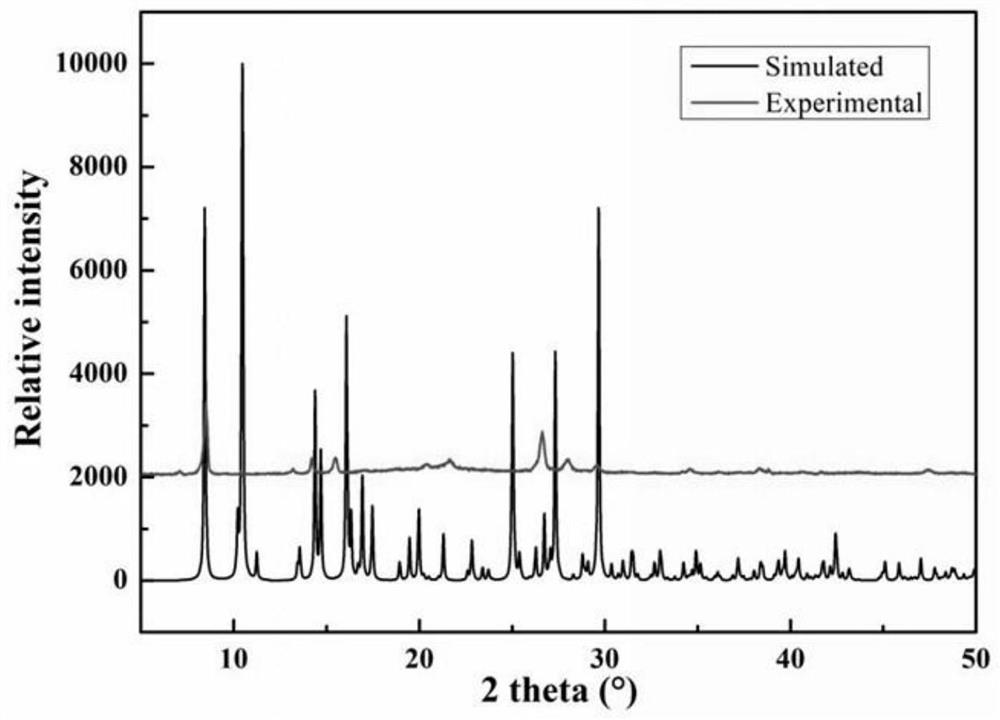
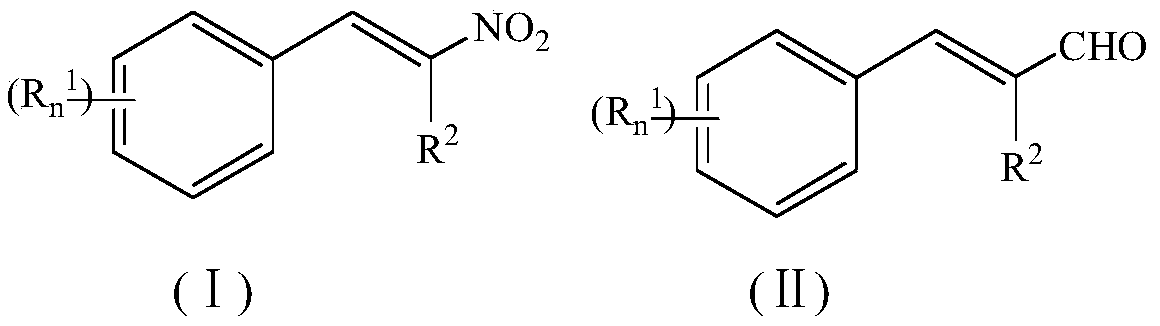
ActiveCN107641086BLow toxicityWon't happenOrganic chemistryOrganic compound preparationSimple Organic CompoundsNitroalkene
The invention relates to a method for synthesizing an organic compound. The method is used for solving the problem of current synthesis of nitroolefin compounds that reaction byproducts are numerous,raw materials are expensive, and some methods are relatively troublesome in aftertreatment. The invention provides a method for preparing a nitroolefin compound. The nitroolefin compound is prepared through subjecting a cinnamyl aldehyde compound, which serves as a starting material, to a reaction with a nitrate and a solvent for 1 to 24 hours at the temperature of 25 DEG C to 150 DEG C. A methodfor direct nitration by using a cheap nitrate is achieved. The method is a new route for synthesizing the nitroolefin compound.
Owner:ZHEJIANG UNIV OF TECH
Process for preparing α-damascone
ActiveUS10150730B2High yieldCheap productionOrganic compound preparationCarbonyl compound preparationNitroxyl radicalsDamascone
The present invention relates to a process for preparing 1-(2,6,6-trimethylcyclohex-2-en-1-yl)but-2-en-1-one, which comprises a) providing 6,10-dimethylundeca-1,5,9-trien-4-ol, b) oxidizing 6,10-dimethylundeca-1,5,9-trien-4-ol provided in step a) with an oxidizing agent in the presence of at least one organic nitroxyl radical, at least one nitrate compound and an inorganic solid to yield 6,10-dimethylundeca-1,5,9-trien-4-one, c) reacting the 6,10-dimethylundeca-1,5,9-trien-4-one obtained in step b) with an acid to yield 1-(2,6,6-trimethylcyclohex-2-en-1-yl)but-2-en-1-one.
Owner:BASF AG
Catalyst for producing olefins by catalytic cracking and its preparation method and application
ActiveCN106552666BReduce the amount of strong acidImprove stabilityMolecular sieve catalystsHydrocarbon by hydrocarbon crackingAlkaline earth metalAlkali metal oxide
The invention discloses a catalyst for preparing alkene through catalytic cracking. The catalyst takes an alkaline-earth-metal-modified MFI molecular sieve as a carrier and heteropoly acid as an active component, wherein in the alkaline-earth-metal-modified MFI molecular sieve, the weight of alkaline earth metal is 0.5-2.5% of that of an MFI molecular sieve, and the weight of the active component is 0.2-4% of that of the carrier. An alkali metal oxide or alkali metal salt is used to modify the catalyst, and the mass of the alkali metal oxide or alkali metal salt is 0.1-4% of that of the catalyst. A preparation method of the catalyst comprises: soaking the molecular sieve with a nitrate solution of alkaline earth metal, drying the soaked material and performing calcination; loading the modified molecular sieve with the active component through adoption of a heteropoly acid aqueous solution, and drying the soaked material; mashing the obtained material, a proper amount of water and a binder, and performing mixing kneading, molding, drying and calcination; and modifying the obtained material with alkali metal oxide or alkali metal salt, drying the modified product, and performing calcination to obtain the final catalyst. The catalyst is high in alkene yield, low in reaction temperature, and good in stability.
Owner:CHINA PETROLEUM & CHEM CORP +1
Supported Ni-Sb catalyst and preparation method and application thereof
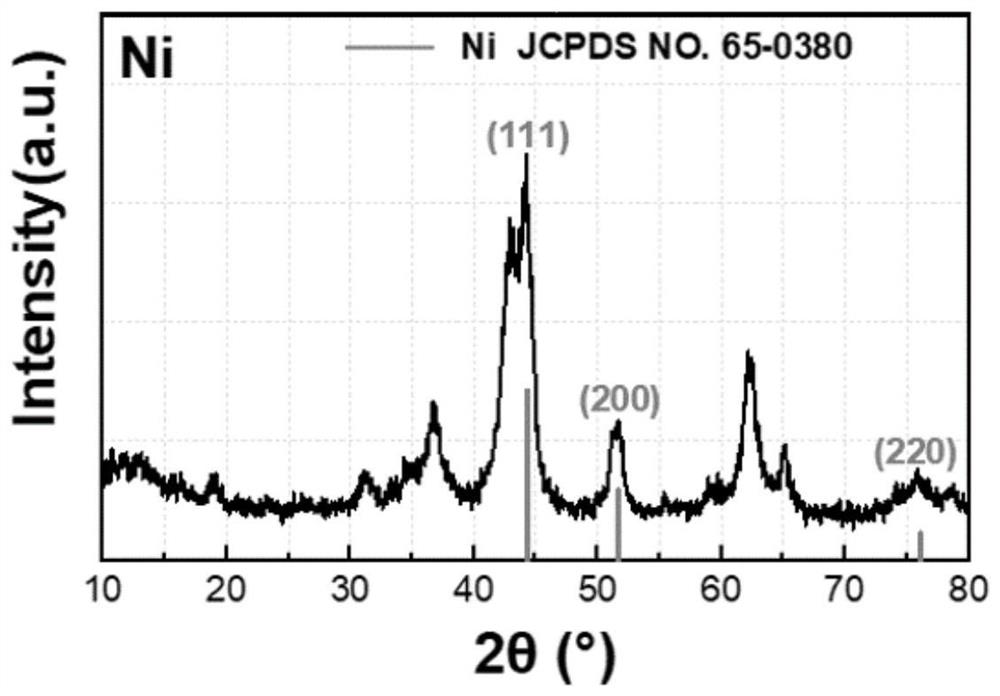
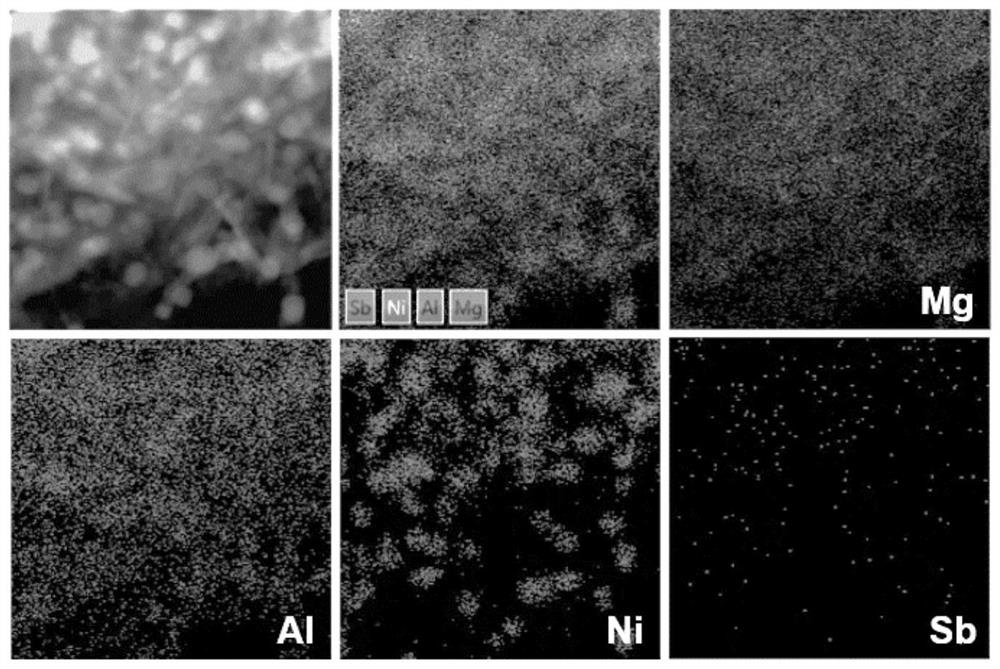
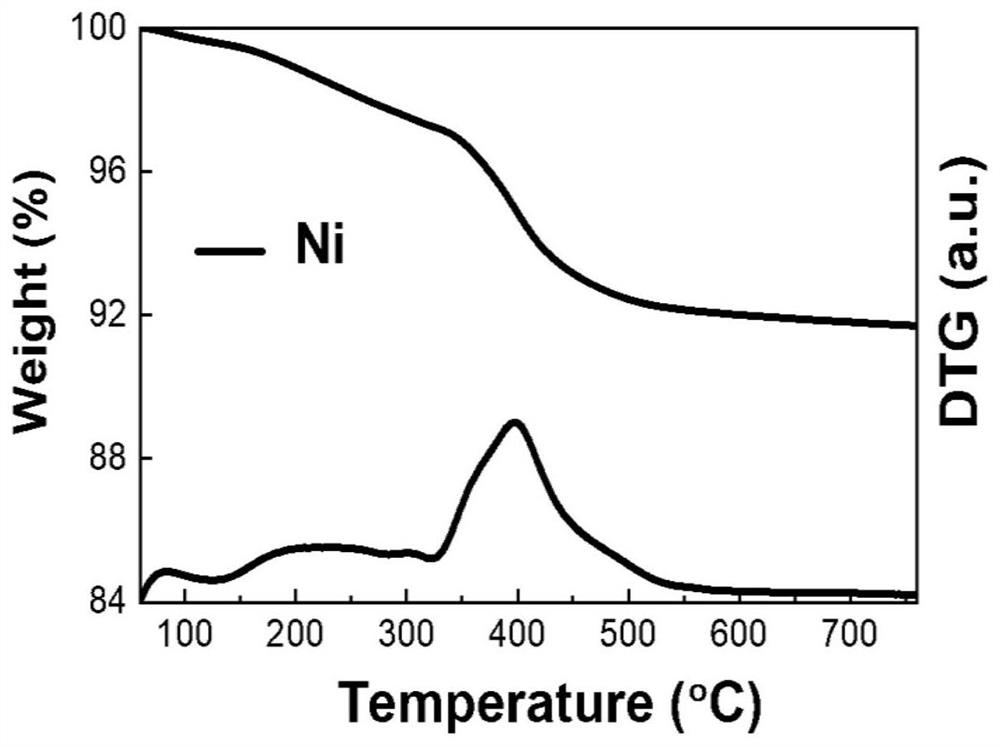
PendingCN114849713AImprove stabilityGood dispersionHydrocarbon by hydrogenationCatalyst activation/preparationPtru catalystAlkyne
The invention discloses a supported Ni-Sb catalyst and a preparation method and application thereof, the catalyst carrier is an aluminum-magnesium metal mixed oxide MgAlOx, the supported metals are Ni and Sb, a hexagonal Ni-Sb intermetallic compound crystal structure is formed, and the active metal Ni site is isolated by the inert metal Sb site. The preparation method comprises the following steps: preparing a mixed metal nitrate solution from corresponding nitrates of metal Ni, Mg and Al, preparing a Ni / Mg / Al ternary layered hydroxide material by adopting a coprecipitation method, mechanically grinding and uniformly mixing antimony powder with the Ni / Mg / Al ternary layered hydroxide material to obtain a Ni / Sb / Mg / Al quaternary layered hydroxide material serving as a Ni-Sb intermetallic compound catalyst precursor; and preparing the supported Ni-Sb intermetallic compound catalyst through a high-temperature hydrogen heat treatment in-situ capture strategy. The catalyst is used in acetylene selective hydrogenation and propyne / propadiene hydrogenation reactions, and shows the characteristics of high ethylene / propylene selectivity and stability under the condition of complete conversion of alkyne / alkadiene.
Owner:EAST CHINA UNIV OF SCI & TECH
Kits of hydralazine compounds and isosorbide dinitrate and/or isosorbide mononitrate
InactiveUS20080226752A1Improving oxygen consumptionImprove exercise toleranceBiocideInorganic active ingredientsVascular diseaseDigitalis
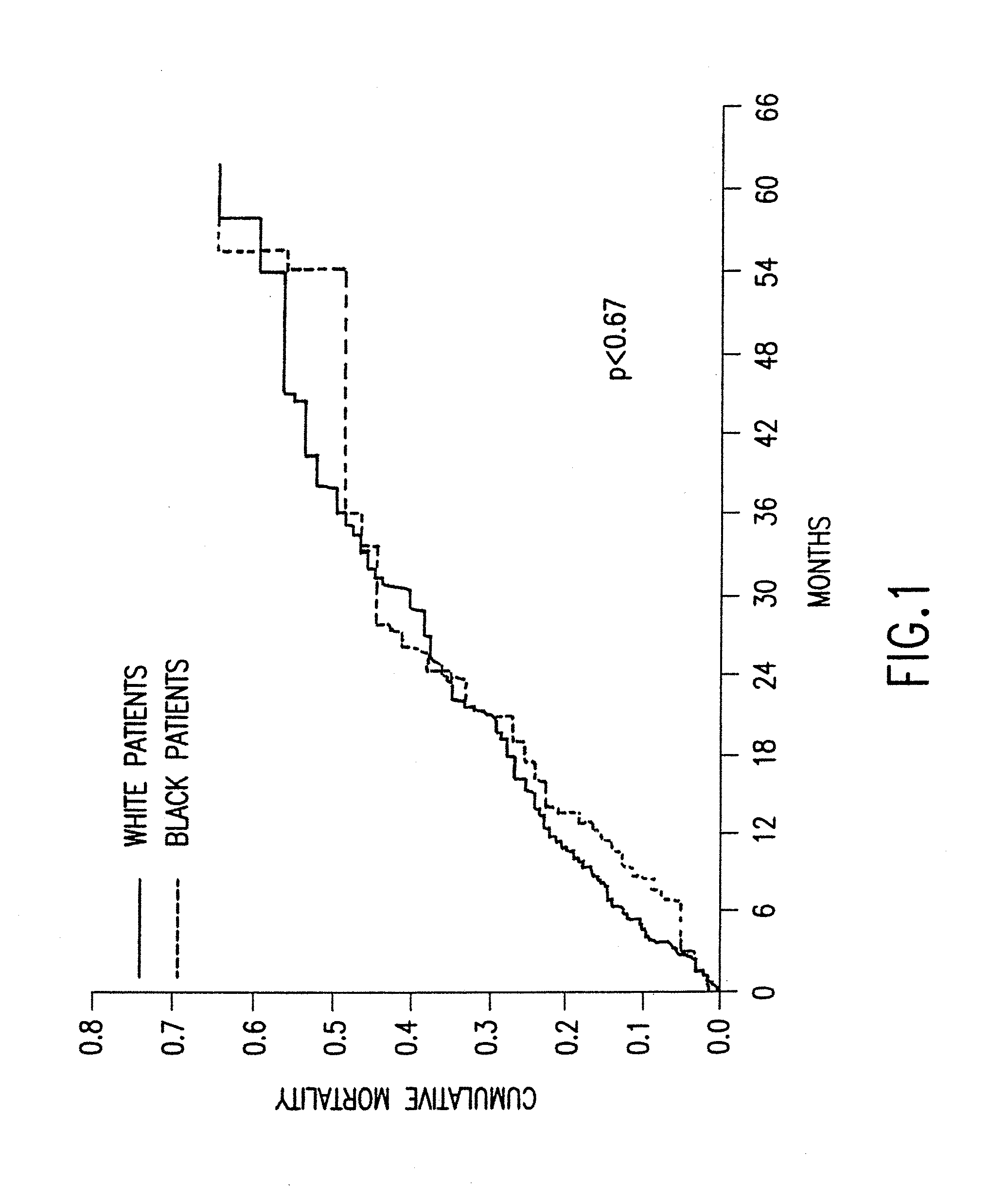
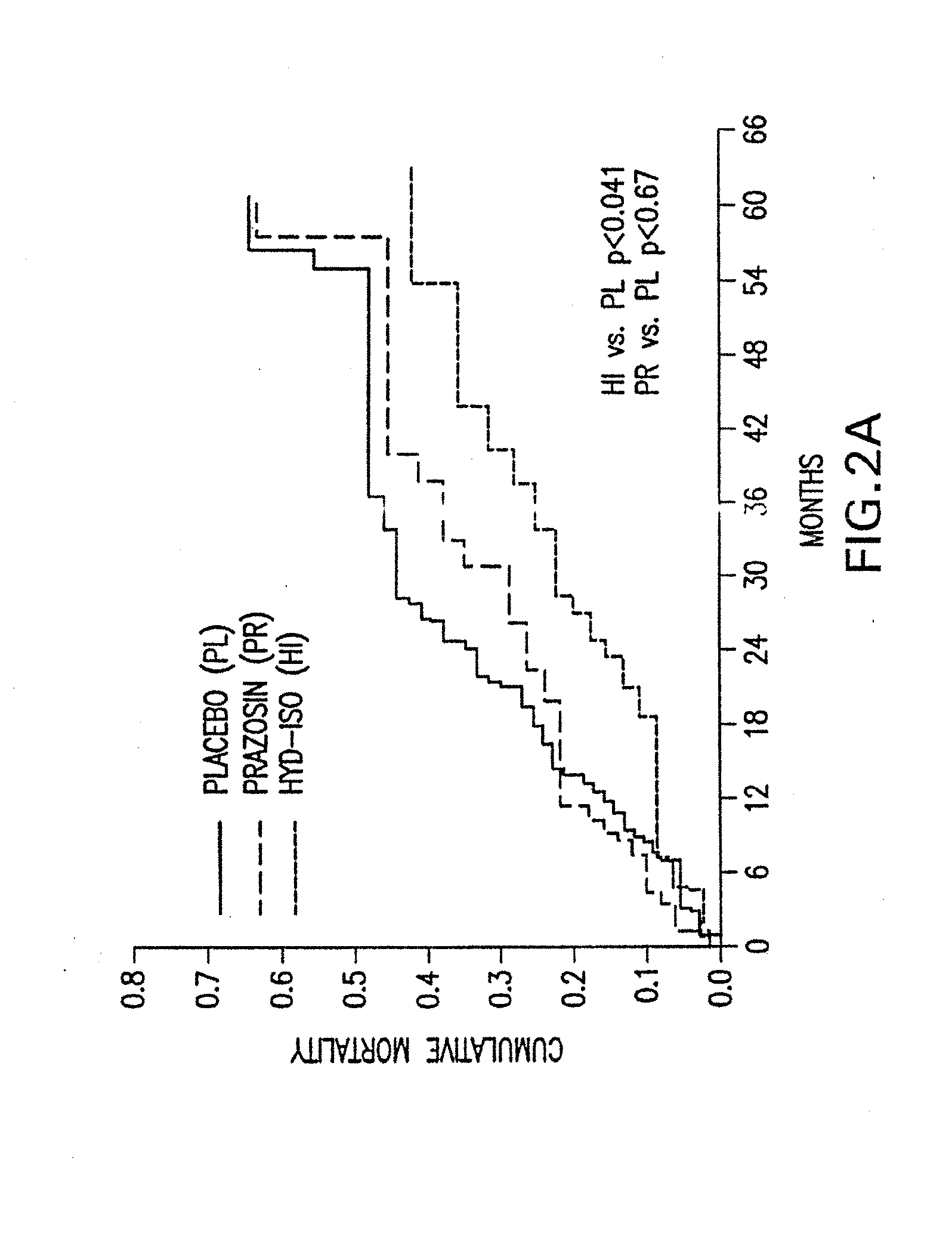
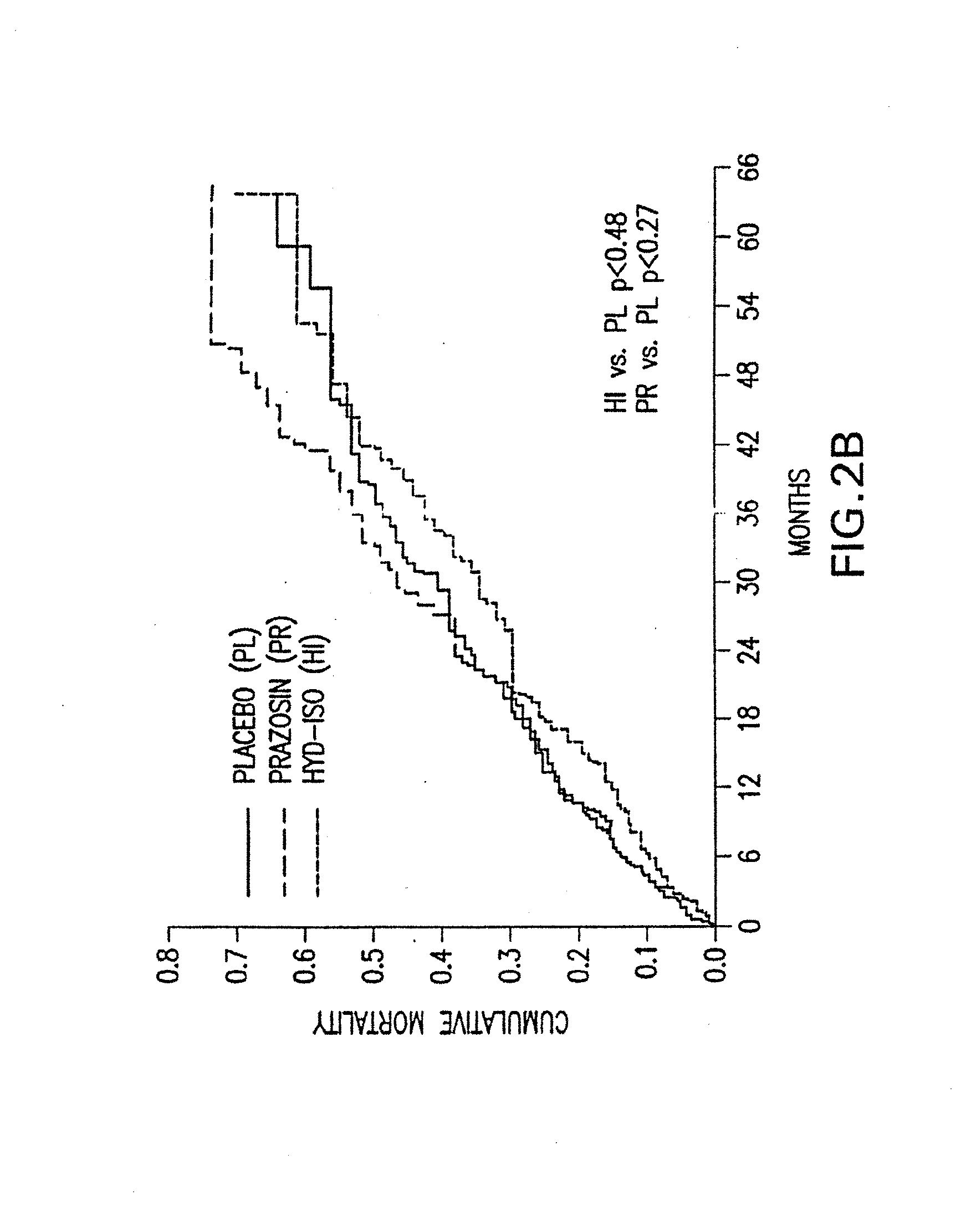
The present invention provides methods of treating and preventing mortality associated with heart failure in an African American patient with hypertension and improving oxygen consumption, quality of life and exercise tolerance by administering a therapeutically effective amount of at least one hydralazine compound and at least one of isosorbide dinitrate and isosorbide mononitrate, and, optionally, one or more compounds, such as, for example, a digitalis, a diuretic compound, or a compound used to treat cardiovascular diseases. In the present invention, the hydralazine compound is preferably hydralazine or a pharmaceutically acceptable salt thereof. Preferred methods of the invention comprise administering hydralazine or a pharmaceutically acceptable salt thereof and isosorbide dinitrate.
Owner:NITROMED
Obligately symbiotic thermophile Symbiobacterium toebii SC-1 producing thermostable L-tyrosine phenol-lyase and L-tryptophan indole-lyase
The present invention relates to a novel obligately symbiotic thermophile Symbiobacterium toebii SC-1 (Accession NO: KCTC 0685BP), and a thermostable L-tyrosine phenol-lyase and L-tryptophan indole-lyase produced by Symbiobacterium toebii SC-1. The present invention also relates to a pure culture method of Symbiobacterium toebii SC-1, a method of growth measurement for Symbiobacterium toebii SC-1 and its relative symbiotic bacteria showing low-growth yield using nitrate respiration, and a screening method of relative symbiotic strains of Symbiobacterium toebii SC-1 from the environment using a specific antibody to it. Therefore, the thermostable L-tyrosine phenol-lyase and L-tryptophan indole-lyase produced by Symbiobacterium toebii SC-1 in this invention can provide more stable catalysts in enzymatic biotransformation processes which enzymatically produce valuable medico-amino acids 3,4-dehydroxy-L-phenylalanine (L-DOPA) and L-tryptophan.
Owner:KOREA RES INST OF BIOSCI & BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com