Spiropyrane coordination compound photochromic material and preparation method thereof
A technology of photochromic materials and complexes, applied in the direction of color-changing fluorescent materials, organic compounds/hydrides/coordination complex catalysts, organic chemical methods, etc., can solve the problem of small changes in absorption spectrum and thermal stability of cis-azobenzene Poor performance, poor thermal stability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
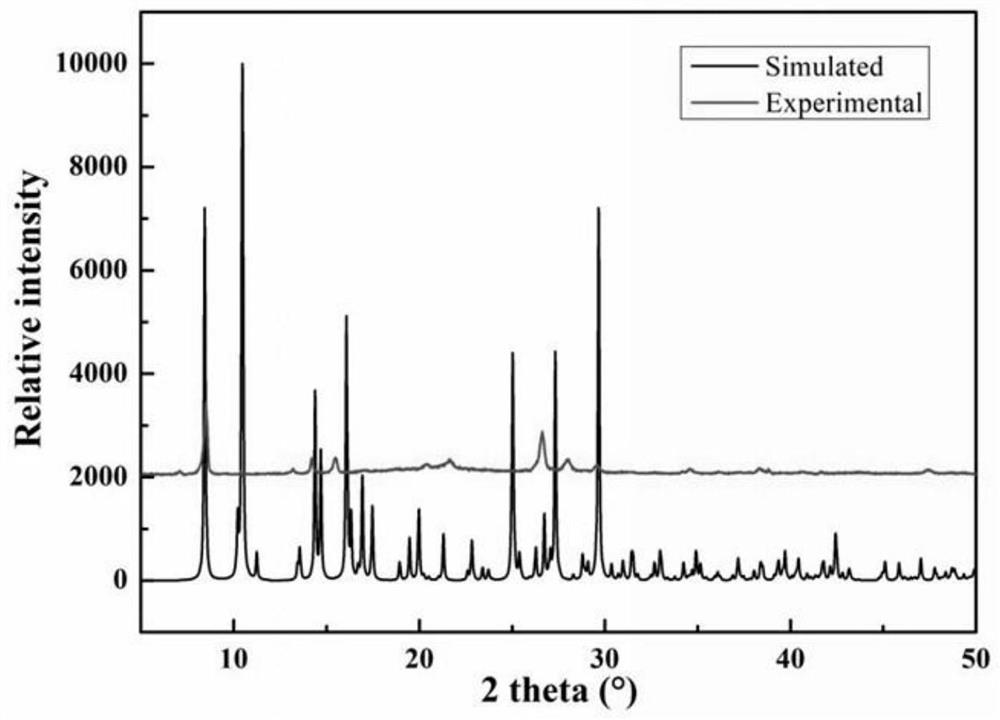
Image
Examples
Embodiment 1
[0039] Spiropyran complex photochromic material, including the following raw materials: nickel nitrate, N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran and ethylenediamine, the molar volume ratio is 0.3mmol: 0.1mmol: 0.1mL;
[0040] The preparation method of the above-mentioned spiropyran complex photochromic material specifically comprises the following steps:
[0041] (1) take each raw material by above-mentioned molar volume ratio;
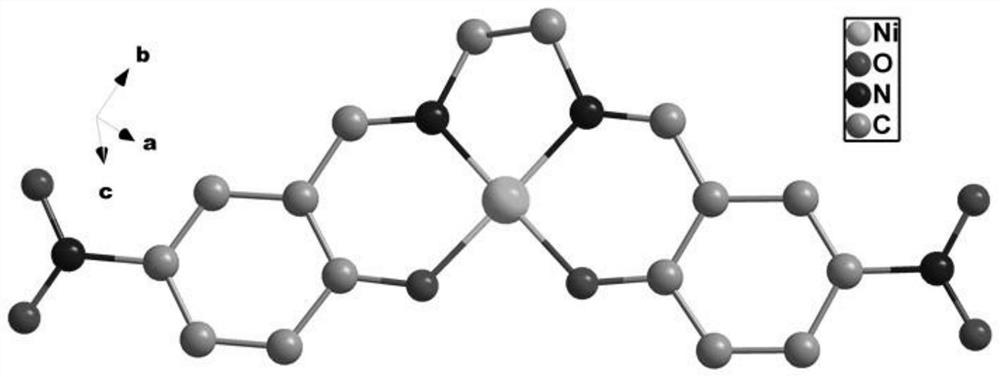
[0042] (2) Add 0.3mmol nickel nitrate, 0.1mmol N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran and 0.1mL aliphatic diamine to 1mL N,N`-dimethyl In formamide and 3mL of n-propanol, place it at a constant temperature at 80°C for 72h to obtain the photochromic material of the spiropyran complex (brown yellow crystal, the structural formula is as follows: figure 1 shown).
Embodiment 2
[0044] A spiropyran complex photochromic material, comprising the following raw materials: copper nitrate, N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran and propylenediamine, with a molar volume ratio of 0.2mmol: 0.2mmol: 0.2mL;
[0045] The preparation method of the above-mentioned spiropyran complex photochromic material, specifically comprises the following steps:
[0046] (1) each raw material is weighed by above-mentioned molar volume ratio;
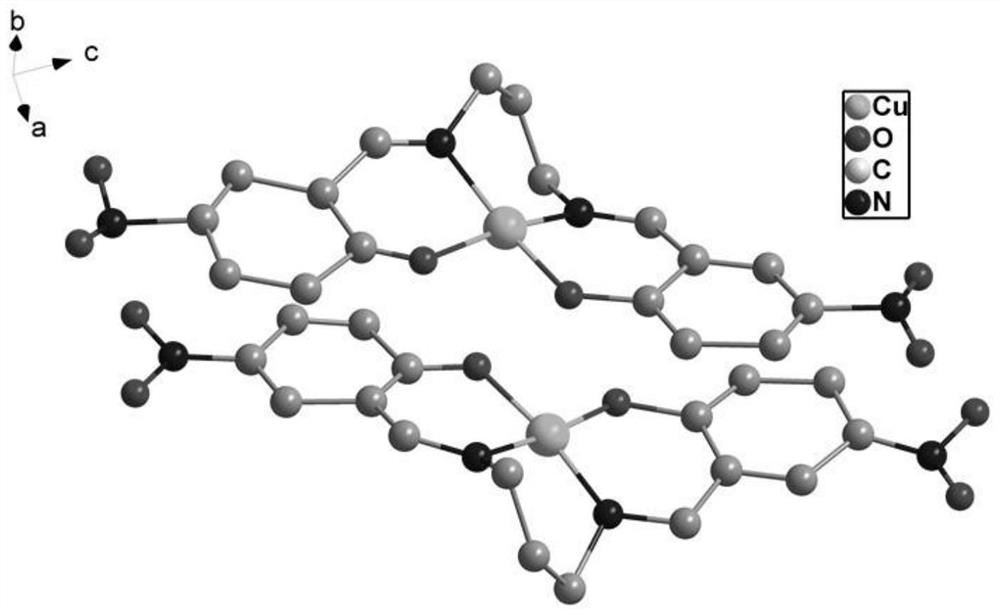
[0047] (2) Add 0.3 mmol copper nitrate, 0.1 mmol N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran and 0.1 mL aliphatic diamine to 2 mL N,N`-dimethylmethane amide and 3 mL of n-propanol, and placed at a constant temperature of 75 ° C for 70 h, to obtain the spiropyran complex photochromic material (brown yellow crystal, the structural formula is as follows: figure 2 shown).
Embodiment 3
[0049] A spiropyran complex photochromic material, comprising the following raw materials: nickel nitrate, N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran and ethylenediamine, with a molar volume ratio of 0.4mmol: 0.1mmol: 0.1mL;
[0050] The preparation method of the above-mentioned spiropyran complex photochromic material, specifically comprises the following steps:
[0051] (1) each raw material is weighed by above-mentioned molar volume ratio;
[0052] (2) Add 0.3 mmol of nickel nitrate, 0.1 mmol of N-hydroxyethyl-3,3-dimethyl-6-nitroindoline spiropyran and 0.1 mL of aliphatic diamine to 1 mL of N,N'-dimethyl The spiropyran complex photochromic material (brown-yellow crystal) was obtained by placing it in formamide and 4 mL of n-propanol at a constant temperature of 80 °C for 75 h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com