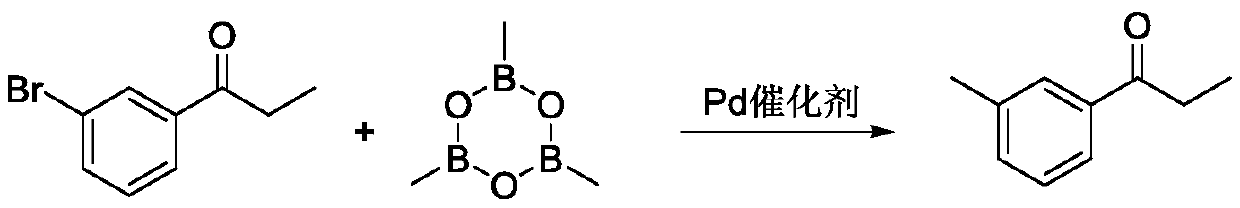
Synthetic method of 3 '-methyl propiophenone
A technology of methyl propiophenone and methyl phenyl propanol, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of hydroxyl compounds, etc., can solve the problems of large consumption of oxidants, serious environmental pollution, and difficulty in recycling and reuse. , to achieve the effect of high yield, good selectivity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
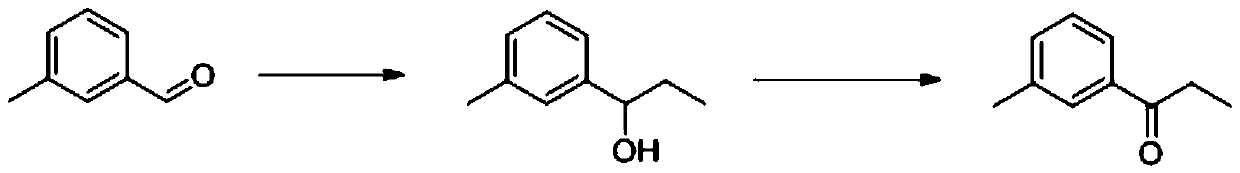
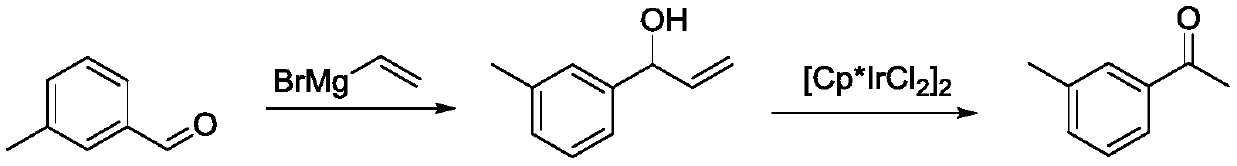
[0037] A 1L three-neck flask containing 1mol / L ethylmagnesium bromide tetrahydrofuran solution (700mL) was placed in an ice-water bath, and a mixture of m-tolualdehyde (0.6mol, 72.09g) and tetrahydrofuran (40mL) was added dropwise thereto. Solution, control the reaction temperature not to exceed 10°C. After the dropwise addition, the reaction was stirred at room temperature for 2 h, and then tetrahydrofuran was recovered by distillation under reduced pressure. The flask was then placed in an ice-water bath, and a 10% by mass hydrochloric acid solution was added dropwise therein, and the temperature was controlled not to exceed 10° C. until the pH of the system was neutral. Stand to separate the layers, separate the organic phase, then extract the aqueous phase with dichloromethane, combine the organic phases and concentrate to obtain a light yellow liquid, namely 3'-methylphenylpropanol. The content of 3'-methylpropanol measured by liquid chromatography is 98%.
[0038] The ...
Embodiment 2
[0040] A 1L three-neck flask equipped with 1mol / L ethylmagnesium chloride tetrahydrofuran solution (600mL) was placed in an ice-water bath, and a mixed solution composed of m-tolualdehyde (0.5mol, 60.08g) and tetrahydrofuran (20mL) was added dropwise thereto, Control the reaction temperature not to exceed 10°C. After the dropwise addition, the reaction was stirred at room temperature for 2 h, and then tetrahydrofuran was recovered by distillation under reduced pressure. The flask was then placed in an ice-water bath, and a 10% by mass hydrochloric acid solution was added dropwise therein, and the temperature was controlled not to exceed 10° C. until the pH of the system was neutral. Stand to separate the layers, separate the organic phase, then extract the aqueous phase with dichloromethane, combine the organic phases and concentrate to obtain a light yellow liquid, namely 3'-methylphenylpropanol. The content of 3'-methylpropanol measured by liquid chromatography is 98%.
[...
Embodiment 3
[0043] A 1L three-neck flask containing 1mol / L ethylmagnesium bromide tetrahydrofuran solution (700mL) was placed in an ice-water bath, and a mixture of m-tolualdehyde (0.6mol, 72.09g) and tetrahydrofuran (40mL) was added dropwise thereto. Solution, control the reaction temperature not to exceed 10°C. After the dropwise addition, the reaction was stirred at room temperature for 2 h, and then tetrahydrofuran was recovered by distillation under reduced pressure. The flask was then placed in an ice-water bath, and a 10% by mass hydrochloric acid solution was added dropwise therein, and the temperature was controlled not to exceed 10° C. until the pH of the system was neutral. Stand to separate the layers, separate the organic phase, then extract the aqueous phase with dichloromethane, combine the organic phases and concentrate to obtain a light yellow liquid, namely 3'-methylphenylpropanol. The content of 3'-methylpropanol measured by liquid chromatography is 98%.
[0044]Add t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com