A kind of preparation method of fluorescent probe
A technology of ratiometric fluorescent probes and compounds, applied in the direction of fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of poor water solubility, complex synthesis, poor selectivity, etc., and achieve sensitive response, simple synthesis, and good stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] (Scheme 1) Dissolve 200mg (0.38mmol) of benzothiazole-rhodamine compounds in 15mL of absolute ethanol, then add 57mg (1.14mmol) of hydrazine hydrate and reflux for 6h, and spin dry the solvent under reduced pressure. The crude product was separated by column chromatography with a mixed system of dichloromethane and methanol (v / v, 100:1) to obtain a pure product, 110 mg of a light yellow pure product with a yield of 54%.
[0033] (Scheme 2) Dissolve 200mg (0.38mmol) of benzothiazole-rhodamine compounds in 15mL of absolute ethanol, then add 95mg (1.9mmol) of hydrazine hydrate and reflux for 6h, and spin dry the solvent under reduced pressure. The crude product was separated by column chromatography with a mixed system of dichloromethane and methanol (v / v, 100:1) to obtain a pure product, 130 mg of a light yellow pure product with a yield of 64%.
[0034] (Scheme 3) 200 mg (0.38 mmol) of benzothiazole-rhodamine compounds were dissolved in 15 mL of absolute etha...
Embodiment 2
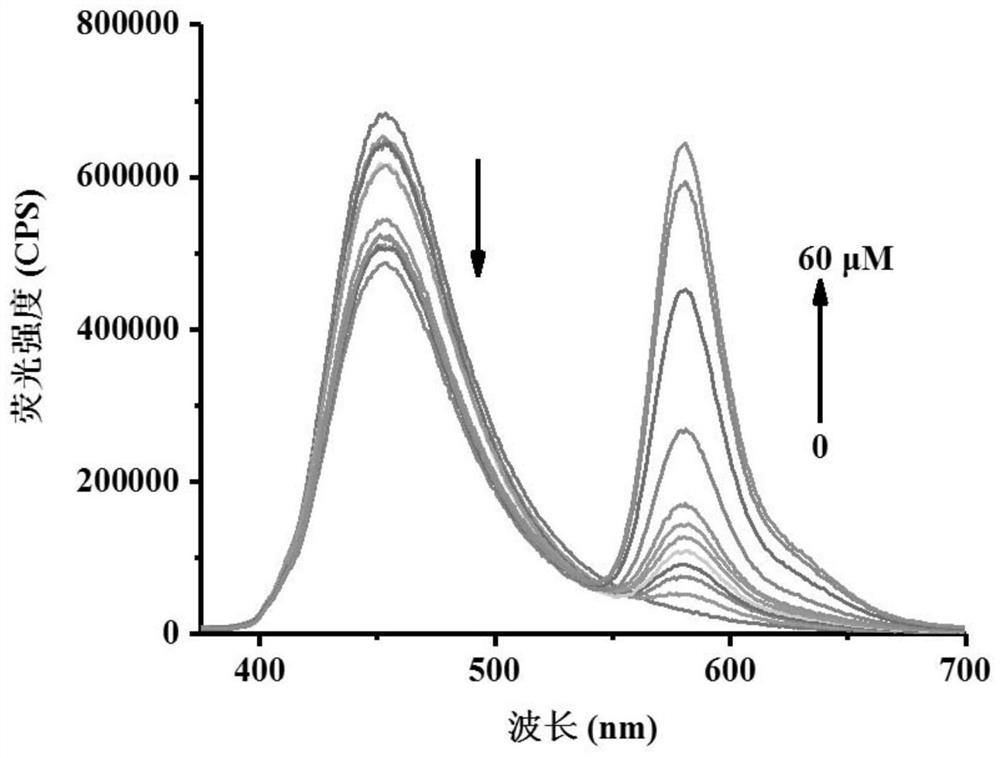
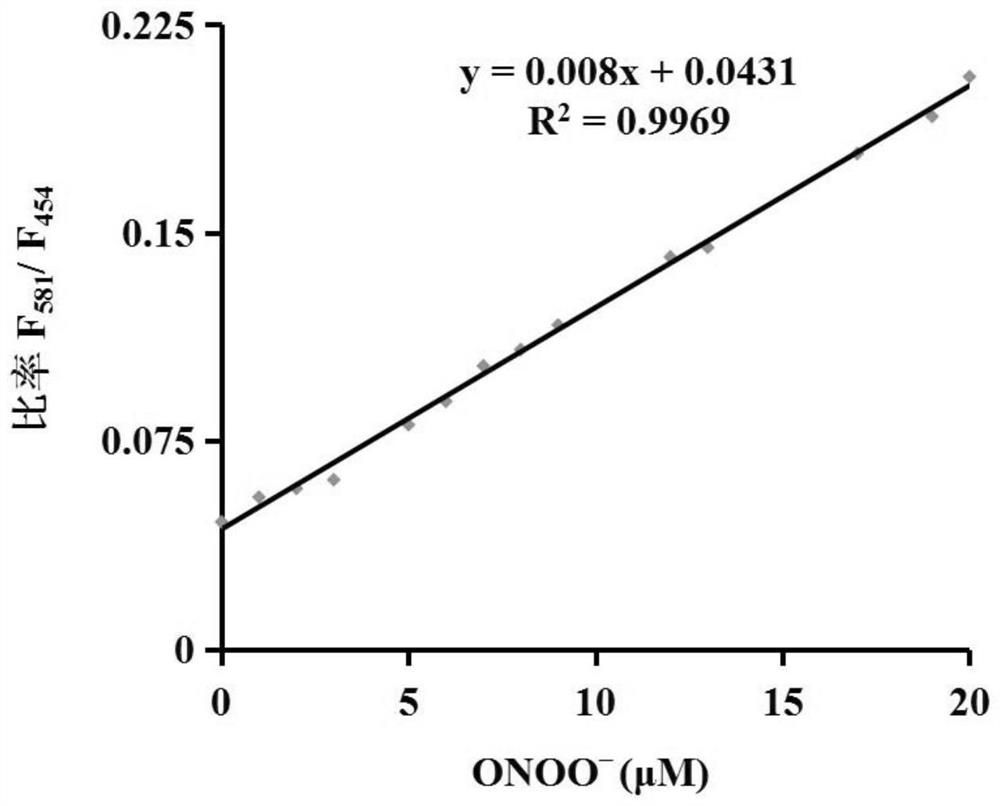
[0039] Figure 1ais the fluorescence spectrum of the probe (5 μM) before and after adding peroxynitrite (0-60 μM). Figure 1b It is a linear relationship graph of different concentrations of peroxynitrite (0-20 μM) to probe (5 μM).
[0040] Configure multiple parallel samples with a probe concentration of 5 μM in a 10mL colorimetric tube, then add different concentrations of peroxynitrite into the test system, shake it evenly and let it stand. The above-mentioned determination is carried out in DMSO: water=3: 7 (20mMPBS, pH 7.4) system, the probe used is the probe prepared in Example 1, and all spectral tests are all at 25 Measured at °C.
[0041] Fluorescence intensity changes were measured with a fluorescence spectrometer, from Figure 1a It can be clearly seen that as the concentration of peroxynitrite increases, the fluorescence intensity at 454 nm decreases, and the fluorescence intensity at 581 nm increases gradually. and, by Figure 1b It can be seen that after the f...
Embodiment 3
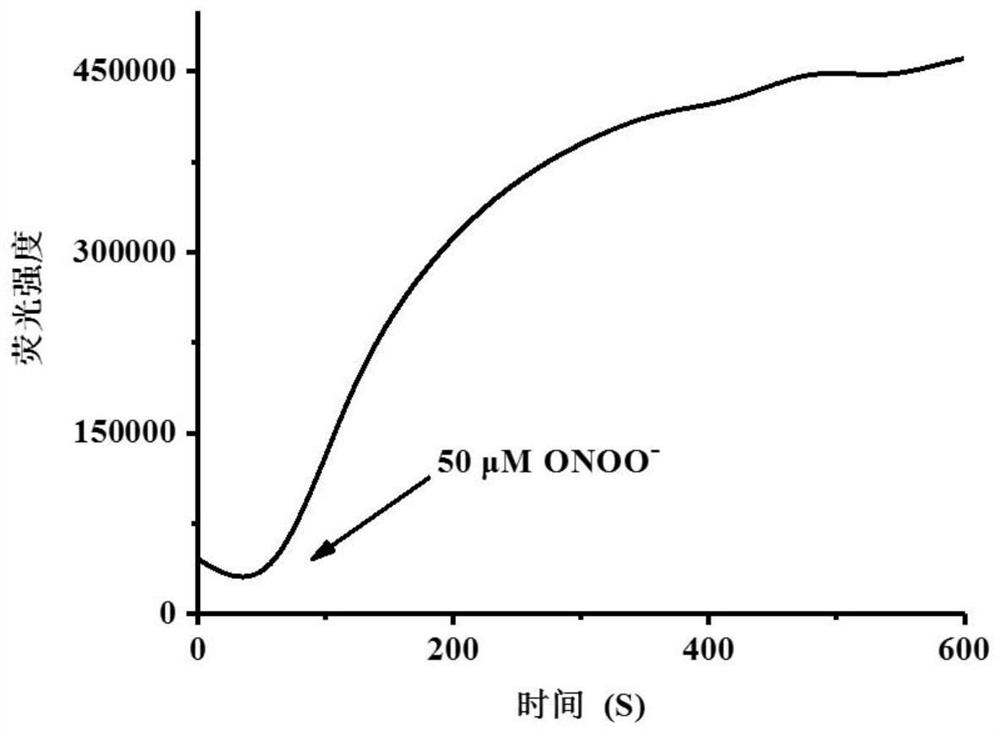
[0043] figure 2 is the response time of the probe (5 μM) after addition of peroxynitrite (50 μM). Take 50 μL from the probe mother solution and place it in a 10 mL test system, then add 50 μM peroxynitrite into the test system, shake it evenly, and measure the change of its fluorescence intensity with a fluorescence spectrometer immediately. The above-mentioned determination is carried out in DMSO: water=3: 7 (20mM PBS, pH7.4) system, the probe used is the probe prepared in Example 1, and all spectral tests are Measured at 25°C.
[0044] It can be clearly seen from the figure that after the addition of peroxynitrite, the fluorescence intensity reaches the maximum value and remains unchanged after detection for about 6 minutes, which indicates that the probe reacts rapidly with peroxynitrite and can be used for peroxynitrite. The determination of nitrate provides a rapid analytical method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com