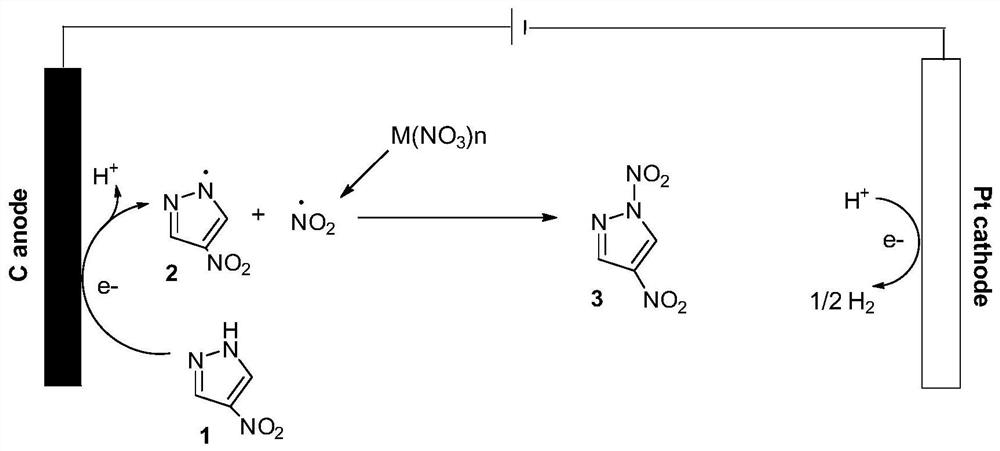
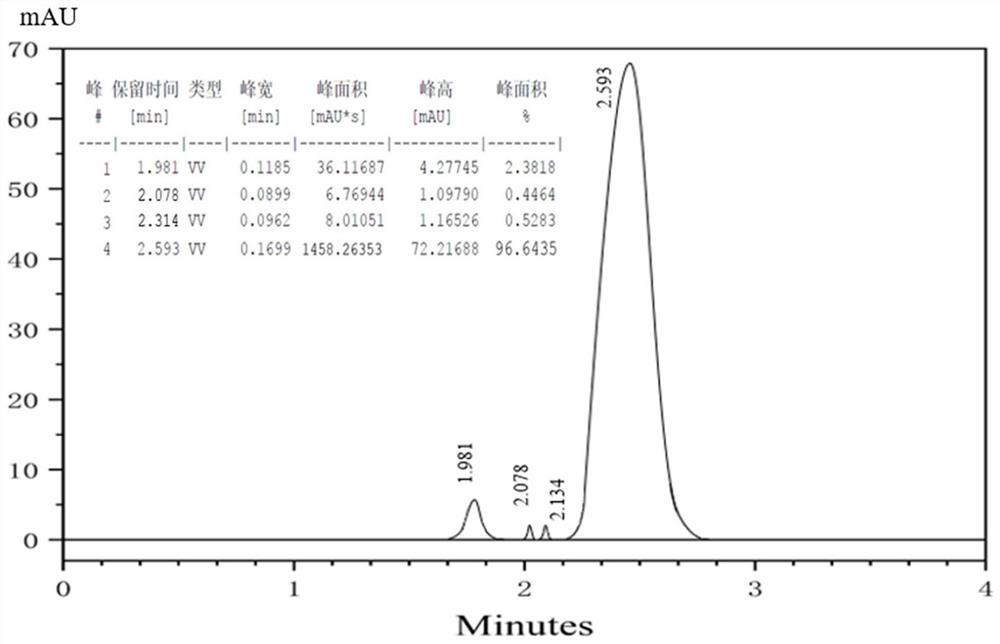
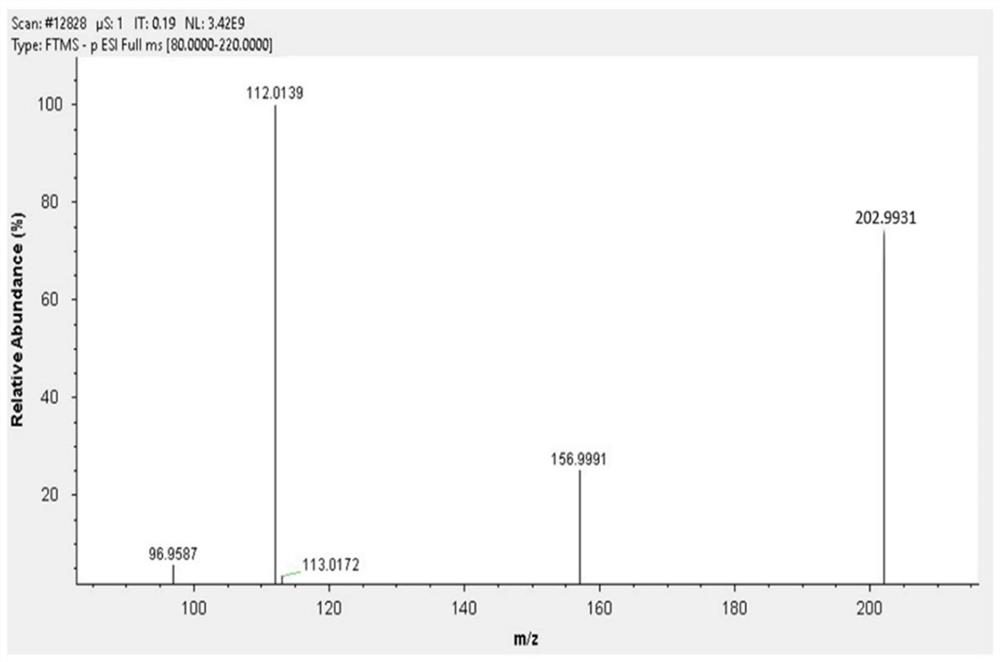
Method for preparing 1,3,5-trinitropyrazole by electrochemical method
A technology for nitropyrazole and dinitropyrazole, which is applied in the field of electrochemical synthesis of 1,3,5-trinitropyrazole, can solve the problems of difficult post-processing, many side reactions, and high reaction cost, and achieves The effect of good substrate universality, high yield and simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026]
[0027] Under stirring at room temperature, slowly add 1.5 g of 1,3-dinitropyrazole into a flask containing 12 mL of o-dichlorobenzene, raise the temperature in the sand bath to 175 ° C, and stir at constant temperature for 8 h. After the reaction is completed, the reaction solution is cooled to Cool in an ice-water bath at room temperature, filter under reduced pressure to obtain the crude product of C-nitropyrazole, add an appropriate amount of diethyl ether to the crude product, reflux and stir for 0.5h, cool down in an ice-water bath and filter, remove diethyl ether in a vacuum, and obtain relatively pure 3,5-nitropyrazole base pyrazole.
Embodiment 2
[0029] Other conditions are the same as in Example 1, and the experiments of different reaction times are checked, and the experimental results are shown in Table 1.
[0030] The influence of table 1 reaction time on productive rate
[0031]
Embodiment 3
[0033] Other conditions are the same as in Example 1, and the experiments of different reaction temperatures are checked, and the experimental results are shown in Table 2.
[0034] The influence of table 2 temperature of reaction on productive rate
[0035]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com