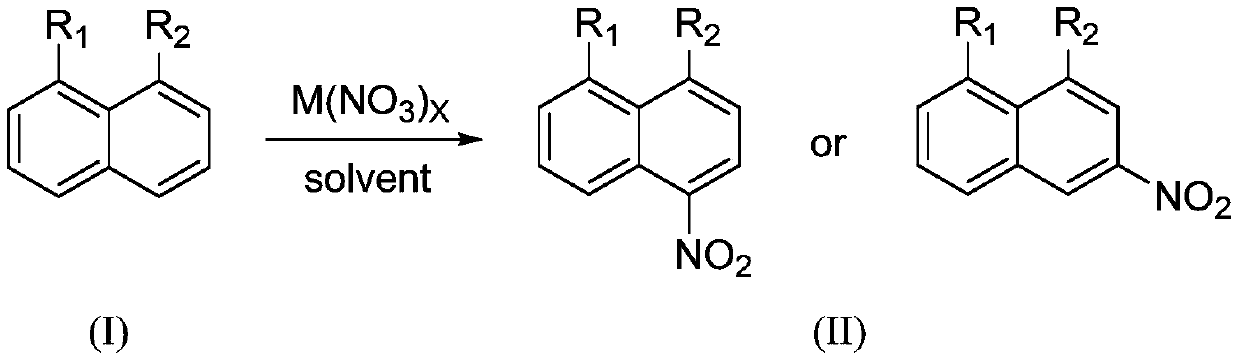
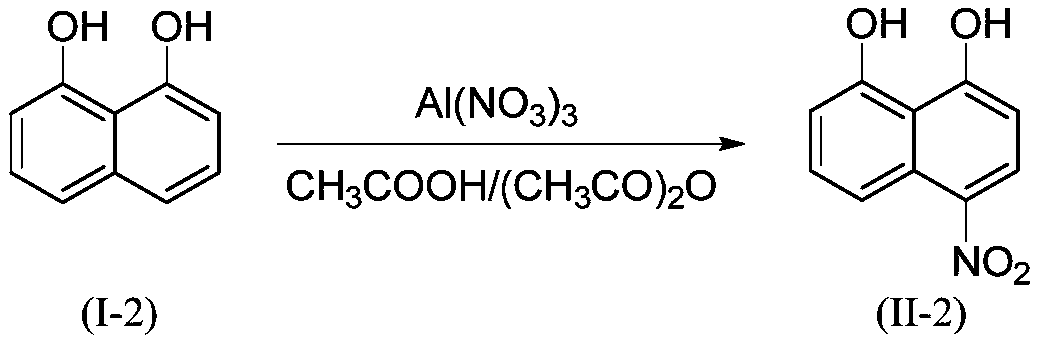Preparation method of 1,8-disubstituted naphthalene mononitration derivative
A disubstituted and derivative technology, applied in the field of preparation of 1,8-disubstituted naphthalene mononitrated derivatives, can solve the problems of difficult application, low substrate universality, environmental pollution, etc., and achieve high product yield , low cost, high purity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
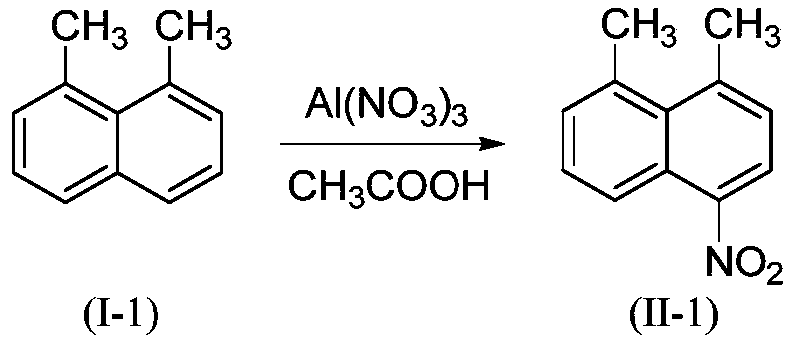
Embodiment 1
[0027]
[0028] 3.12g (20mmol, M=156.23) 1,8-dimethylnaphthalene (I-1), 9.00g (24 mmol, M=375.13) Al(NO 3 ) 3 .9H 2 O was added to 30 mL CH 3 In COOH, the temperature was controlled at 40°C for 6 hours, and the end point of the reaction was monitored by TLC until the disappearance of the raw material point. Cool to room temperature, filter with suction, wash with 5 ~ 10mL H 2 O and anhydrous C 2 h 5 The filter cake was washed with OH and vacuum dried to obtain the light yellow product 4-nitro-1,8-dimethylnaphthalene (II-1) with a yield of 91%. HPLC purity 98.5%. HRMS(ES+)C 12 h 12 NO 2 ([M+H]) + The theoretical value is 202.0868, and the measured value is 202.0868.
Embodiment 2
[0030]
[0031] 3.20g (20mmol, M=160.17) 1,8-dihydroxynaphthalene (I-2), 9.00g (24 mmol, M=375.13) Al(NO 3 ) 3 .9H 2 O was added to 15mL CH 3 COOH+15mL (CH 3 CO) 2 In O, the temperature was controlled at 20°C for 6 hours, and the other conditions and preparation steps were the same as in Example 1 to obtain the orange-yellow product 4-nitro-1,8-naphthalenediol (II-2), with a yield of 90%. HPLC purity 99.0%. HRMS(ES+)C 10 h 8 NO 4 ([M+H]) + The theoretical value is 206.0453, and the measured value is 206.0451.
Embodiment 3
[0033]
[0034] 3.16g (20mmol, M=158.20) 1,8-diaminonaphthalene (I-3), 9.48g (24 mmol, M=394.98) Bi(NO 3 ) 3 Added to 15mL CH 3 COOH+15mL (CH 3 CO) 2 In O, the temperature was controlled at 20°C for 6 hours. Other conditions and preparation steps were the same as in Example 1 to obtain the orange-red product 4-nitro-1,8-naphthalenediamine (II-3) with a yield of 93%. HPLC purity 99.0%. HRMS(ES+)C 10 h 10 N 3 o 2 ([M+H]) + The theoretical value is 204.0773, and the measured value is 204.0772.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com