Compositions for fecal microbiota transplantation and methods for making and using them and devices for delivering them
A kind of composition, technology of fecal microorganism, applied in non-specific abdominal pain, preparation field, can solve the problem such as limitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
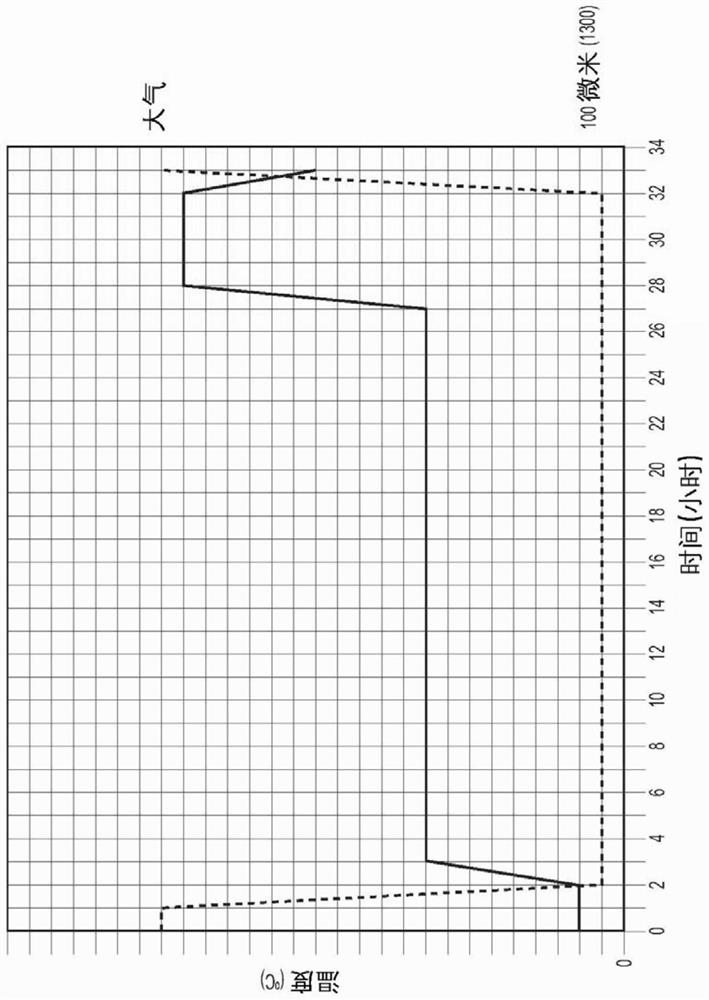
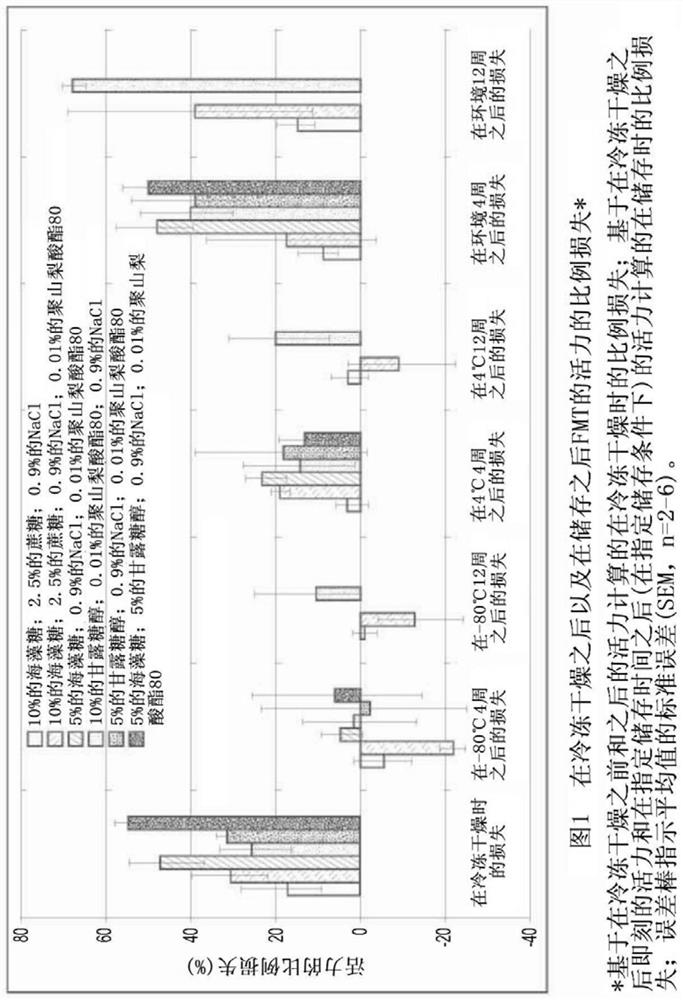
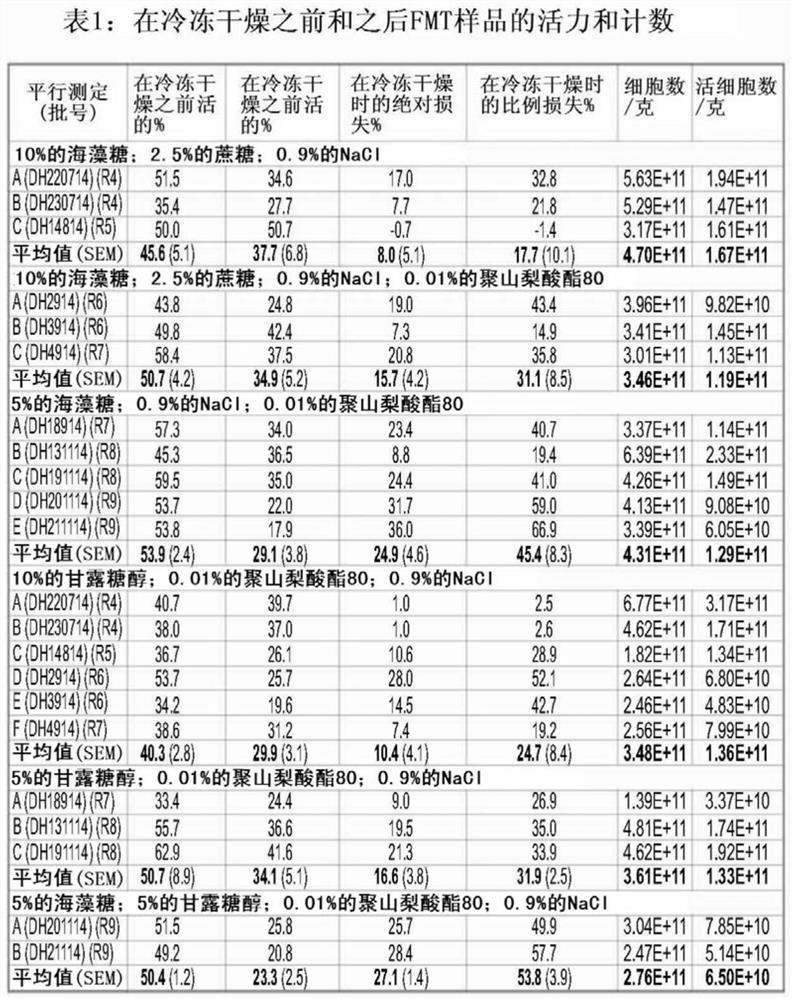
Image
Examples
Embodiment approach 1
[0302] Embodiment 1: A pharmaceutical composition comprising a fecal microbiota preparation in a lyophilized formulation, wherein after storage at ambient temperature or lower for at least 12 weeks, relative to at the beginning of said storage The initial cell viability of the fecal microbiota preparation is capable of maintaining at least 60% of the cell viability.
Embodiment approach 2
[0303] Embodiment 2: The pharmaceutical composition of Embodiment 1, wherein after storage at ambient temperature or lower for at least 12 weeks, the fecal microbiota preparation is capable of maintaining about 60 % to about 80% cell viability.
Embodiment approach 3
[0304] Embodiment 3: The pharmaceutical composition of Embodiment 1 or 2, wherein the lyophilized fecal microbiota preparation comprises a non-selected and substantially intact fecal microbiota preparation from a single donor.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com