Indole or azaindole derivatives as well as preparation method and application thereof
A technology of indoles and derivatives, applied in the field of medicine, can solve the problems of weak pharmacodynamic activity, high nephrotoxicity, large dose and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12
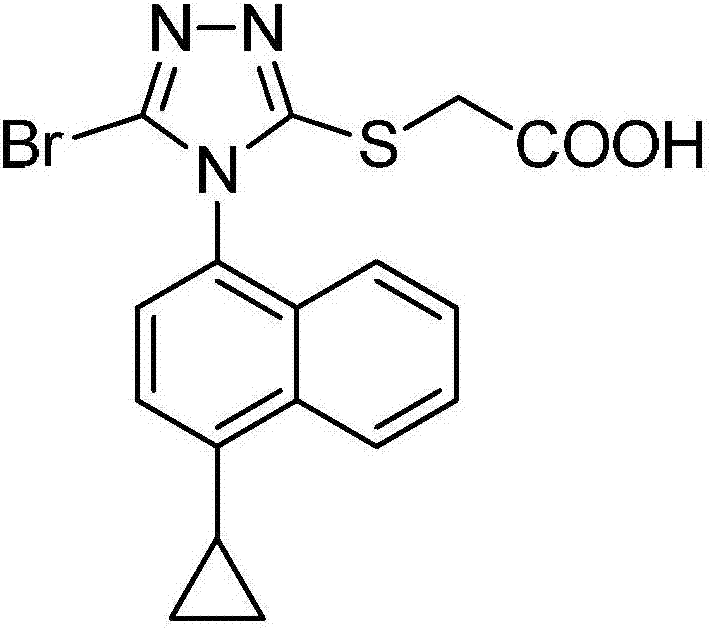
[0200] Example 1 2-[[1-(4-cyanonaphthalen-1-yl)-1H-indol-3-yl]mercapto]acetic acid (I1)
[0201]
[0202] Step 1: 2-[(1H-Indol-3-yl)mercapto]acetic acid
[0203] Indole (2.9g) was dissolved in methanol (30mL), thiourea (12g) was added, a mixed aqueous solution (50mL) of iodine (6.4g) and potassium iodide (4.2g) was added dropwise at room temperature, and the reaction was stirred at room temperature, TLC Monitor the completion of the reaction. Add NaOH solution (2M, 125mL), and stir at 100oC for 10min. Add methyl bromoacetate (4.9g), and stir the reaction at room temperature for 2h. After the TLC reaction was complete. Extract with ethyl acetate (300mL) and water (100mL), wash the ethyl acetate phase with saturated brine (100mL*4), dry the organic phase over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and purify by column chromatography (eluent , dichloromethane:methanol=10:1, v:v), 800 mg of pure product was obtained, and the yield was 15.6%. ...
Embodiment 2
[0207] Example 2 Sodium 2-[[1-(4-cyanonaphthalen-1-yl)-1H-indol-3-yl]mercapto]acetate (I2)
[0208] Dissolve 2-[[1-(4-cyanonaphthalen-1-yl)-1H-indol-3-yl]mercapto]acetic acid (100mg, 0.279mmol) in 5mL methanol, add sodium hydroxide solution (1M , 0.279mL), stirred at room temperature for 10 minutes, concentrated to dryness under reduced pressure to obtain 106 mg of white solid, yield 100%, LC-MS: m / z 359.1 [M+H] + .
Embodiment 32
[0209] Example 3 2-[[1-(4-cyanonaphthalen-1-yl)-1H-pyrrolo[2,3-b]pyridin-3-yl]mercapto]acetic acid (I3)
[0210] Using 7-azaindole as raw material instead of indole, the synthesis method is the same as the preparation method of the compound described in Example 1, LC-MS: m / z 360.1 [M+H] + .
[0211] 1 H NMR (400MHz, DMSO-d 6 ): δ=12.70(s,1H),8.54(d,J=6,4Hz,1H),8.51(t,J=7.6Hz,1H),8.36(d,J=8.4Hz,1H),8.12( s,1H),7.98(d,J=7.6Hz,1H),7.81(d,J=8.0Hz,1H),7.72(t,J=8.0Hz,1H),7.50(t,J=8.0Hz, 1H), 7.45(d, J=6.4Hz, 1H), 7.32(d, J=7.6Hz, 1H), 3.79(s, 2H).
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap