Method for separating uranium from mixture of uranium dioxide and lanthanide oxide
A lanthanide, uranium dioxide technology, applied in electrodes, electrolysis process, electrolysis components, etc., can solve problems such as poor separation effect, and achieve the effect of simplifying the treatment process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] (1) Analytical pure anhydrous LiCl 50g, KCl 50g, mix well, dry and dehydrate and place in a corundum crucible.
[0042] (2) Put the corundum crucible with salt in (1) in a high-temperature resistance furnace, and the high-temperature resistance furnace is placed in a glove box. The glove box controls the water and oxygen content to be less than 1ppm, and then heats to 550°C and melts.
[0043] (3) Add 0.23g UO 2 powder and 0.5g La 2 o 3 At the same time, add to the molten salt in (2), then add 3.0gAlCl 3 , Bubbled high-purity Ar gas and stirred for 10 hours to make the reaction fully proceed.
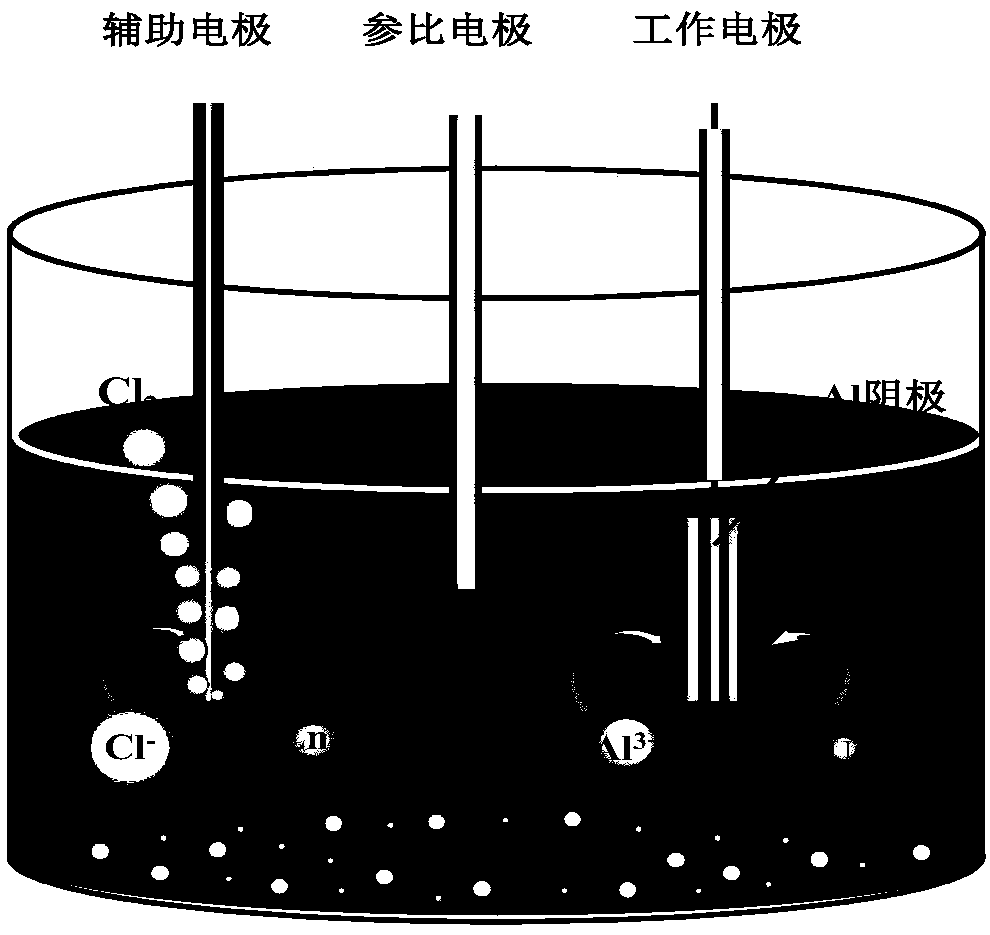
[0044] (4) On the basis of (3), the graphite rod is used as the auxiliary electrode, the Ag / AgCl is used as the reference electrode, and the aluminum sheet electrode is used as the working electrode. A potential of -1.20V is applied to the working electrode to achieve constant potential separation. At the same time, the square wave voltammetry curve is scanned, and the degree...
Embodiment 2
[0048] (1) Analytical pure anhydrous LiCl 45g, KCl 55g, mix well, dry and dehydrate and place in a corundum crucible.
[0049] (2) Put the corundum crucible with salt in (1) in a high-temperature resistance furnace, and the high-temperature resistance furnace is placed in a glove box. The glove box controls the water and oxygen content to be less than 1ppm, and then heats to 500°C and melts.
[0050] (3) Add 0.3g UO 2 powder and 0.5g Nd 2 o 3 At the same time, add to the molten salt in (2), then add 3.0gAlCl 3 , Bubbled high-purity Ar gas and stirred for 10 hours to make the reaction fully proceed.
[0051] (4) On the basis of (3), the graphite rod is used as the auxiliary electrode, the Ag / AgCl is used as the reference electrode, and the aluminum sheet electrode is used as the working electrode. A potential of -1.25V is applied to the working electrode to achieve constant potential separation.
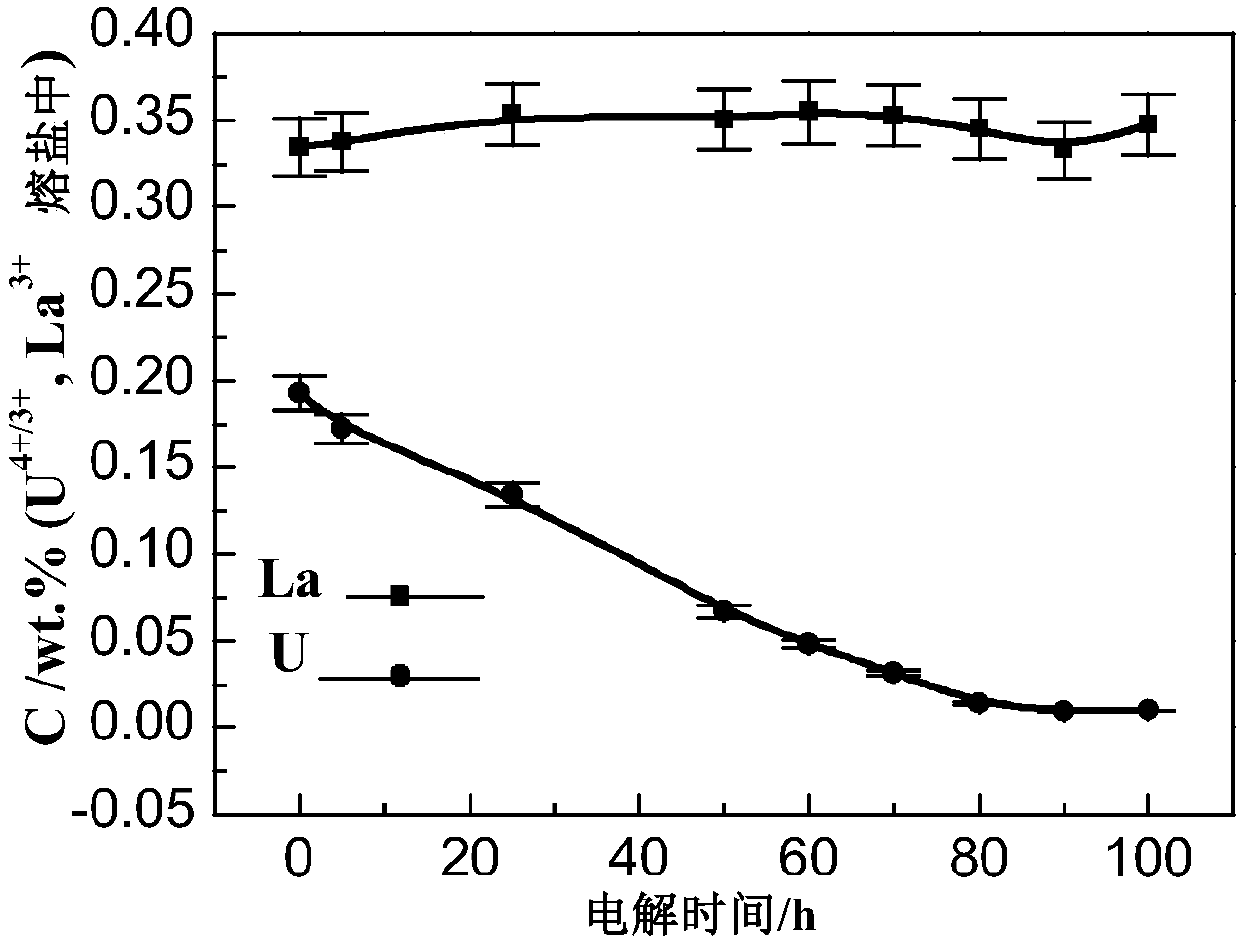
[0052] (5) Take out a small amount of salt in the separation process, and ana...
Embodiment 3
[0056] (1) Analytical pure anhydrous LiCl 44.8g KCl 55.2g, mix well, dry and dehydrate and place in a corundum crucible.
[0057] (2) Put the corundum crucible with salt in (1) in a high-temperature resistance furnace, and the high-temperature resistance furnace is placed in a glove box. The glove box controls the water and oxygen content to be less than 1ppm, and then heats to 550°C and melts.
[0058] (3) 2.18g UO 2 powder and 0.20g La 2 o 3 ,0.20g CeO 2 ,0.20g Sm 2 o 3 At the same time, add in the molten salt melted in (2), then add 6.0g AlCl 3 , Bubbled high-purity Ar gas and stirred for 10 hours to make the reaction fully proceed.
[0059] (4) On the basis of (3), the graphite rod is used as the auxiliary electrode, the Ag / AgCl is used as the reference electrode, and the aluminum sheet electrode is used as the working electrode. A potential of -1.22V is applied to the working electrode to achieve constant potential separation.
[0060] (5) Take out a small amount o...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap