Group I serotype 4 avian adenovirus genetically engineered subunit vaccine, preparation method and application thereof
A subunit vaccine and avian adenovirus technology, which is applied in the field of genetic engineering, can solve the problem of lack of genetic engineering subunit vaccine of group I serotype 4 avian adenovirus, achieve good prevention and control effects, improve immunogenicity, and effectively The effect of immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Construction and preparation of embodiment 1 recombinant baculovirus
[0028] 1. Obtaining gene fragments
[0029] (1) Synthesis of fusion gene fragments
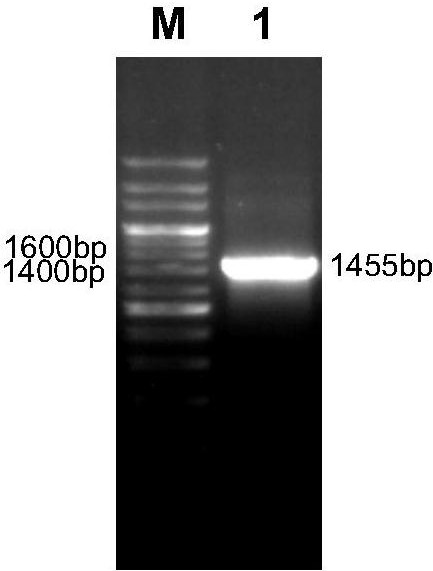
[0030] Analyze the important antigenic sites of group I serotype 4 avian adenovirus fiber and hexon, design the fusion gene of subunit vaccine antigen (amino acid sequence shown in SEQ ID NO: 2), and optimize codons, the sequence is shown in SEQ ID NO:1 shown. Add a BamH I restriction site (GGATCC) at the 5' end of the fusion gene (referred to as FH) as shown in SEQ ID NO:1 in nucleotide sequence, and add a Hind III restriction site (AAGCTT) at the 3' end , synthesized by BGI Corporation.
[0031] (2) Amplify hexon protein and fiber protein coding genes
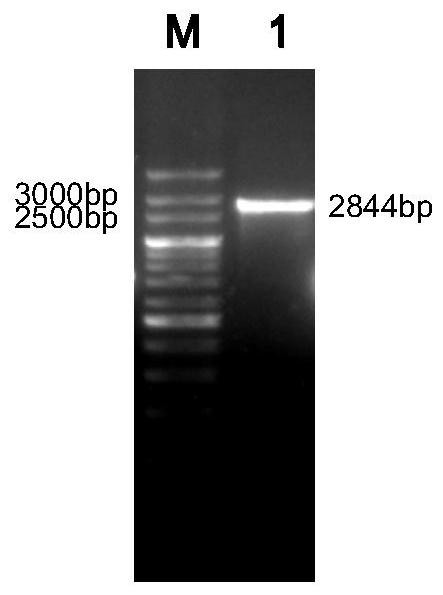
[0032] ①According to the hexon protein coding gene (Genebank accession number: EU938324.1) and fiber protein coding gene (Genebank accession number: HE649966.1) of group I serotype 4 avian adenovirus published by Genbank, designed and synthesized by GenScript P...
Embodiment 2
[0056] Cell culture and titer determination of embodiment 2 recombinant baculovirus
[0057] 1. Preparation of Vaccine Antigens
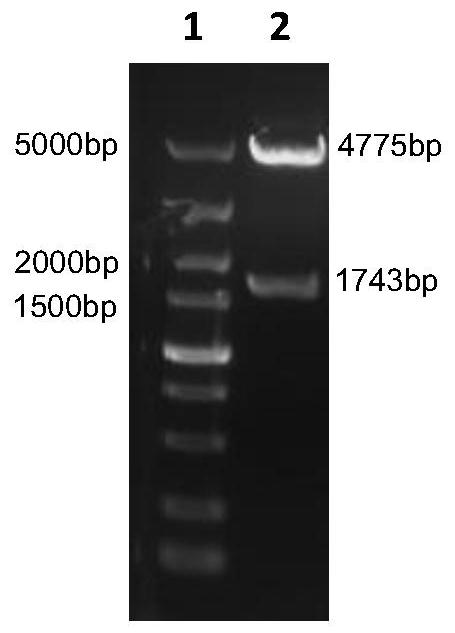
[0058] High Five cells are suspended and cultured in a serum-free medium in a bioreactor to prepare vaccine virus liquid. When the High Five cell density reaches 2×10 6 When cell / mL, the recombinant baculovirus rBV-F-H seed virus was added to the cells according to MOI=1 for cultivation, the saturated oxygen dissolved in the reactor was 40-60%, the stirring speed was 100rpm, the pH was 6.0-6.5, and the temperature was 27°C. After inoculation, samples were taken every 24 hours to observe the lesions of the cells. When the culture time reached 72-96 hours, most of the cells showed lesions (swelling, broken), and the dissolved oxygen value showed a significant upward trend. Cleavage, centrifugation, and supernatant were taken to obtain a fusion protein encoded by the nucleotide sequence shown in SEQ ID NO:1, which was named F-H fusion protein.
[0...
Embodiment 3
[0077] The preparation of embodiment 3 oil adjuvant vaccine
[0078] The F-H fusion protein, recombinant fiber protein, and recombinant hexon protein prepared in Example 2 were tested for sterility according to Appendix 301 of the "Quality Standards for Veterinary Biological Products of the People's Republic of China". Results: There was no bacterial growth.
[0079] Prepare the oil adjuvant vaccine (abbreviated as F-H vaccine) with F-H fusion protein as antigen, comprising the following steps:
[0080] (1) Prepare the oil phase. Prepare the oil phase according to the method on page 343 of the appendix of "Quality Standards for Veterinary Biological Products of the People's Republic of China". The oil phase is prepared from 96 parts by mass of white oil for injection, 4 parts by mass of Siben-80, and 2 parts by mass of aluminum stearate. Take aluminum stearate, mix with a small amount of white oil for injection, heat and melt until translucent, then mix evenly with the full...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com