Motile sperm domain-containing protein 2 and cancer
A technology for cancer and cancer metastasis, applied in the direction of anti-animal/human immunoglobulin, peptide/protein components, medical preparations containing active ingredients, etc., can solve the problem that the biological function of MOSPD2 has not yet been described
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0351] anti-MOSPD2 antibody
[0352] Anti-MOSPD2 polyclonal antibodies were generated according to the following method.
[0353] Materials and methods
[0354] Production and purification of hemagglutinin (HA)-tagged recombinant human MOSPD2 (HA-rhMOSPD2)
[0355]The full-length human MOSPD2 cDNA was inserted into the lentiviral plasmid vector pLVX-EF1α-IRES-Puro (Clonetech, CA) using EcoRI and Xbal restriction sites. An HA-tag encoding oligonucleotide (YPYDVPDYA; SEQ ID NO: 15) was inserted into the N-terminal region of MOSPD2 using an EcoRI restriction site. For transduction, A2058 melanoma cells (ATCC CRL-11147, VA) were spun at 2000 rpm at room temperature in the presence of 8 μg / ml polybrene (Sigma, Israel) and lentiviral particles containing a vector expressing HA-rhMOSPD2 60 minutes. Cells were then seeded in 6-well plates. After 72 hours, fresh medium containing puromycin (4 μg / ml Sigma, Israel) was added for selection of transduced cells. To purify HA-rhMOSPD2,...
Embodiment 2
[0362] MOSPD2 and migration of metastatic cell lines
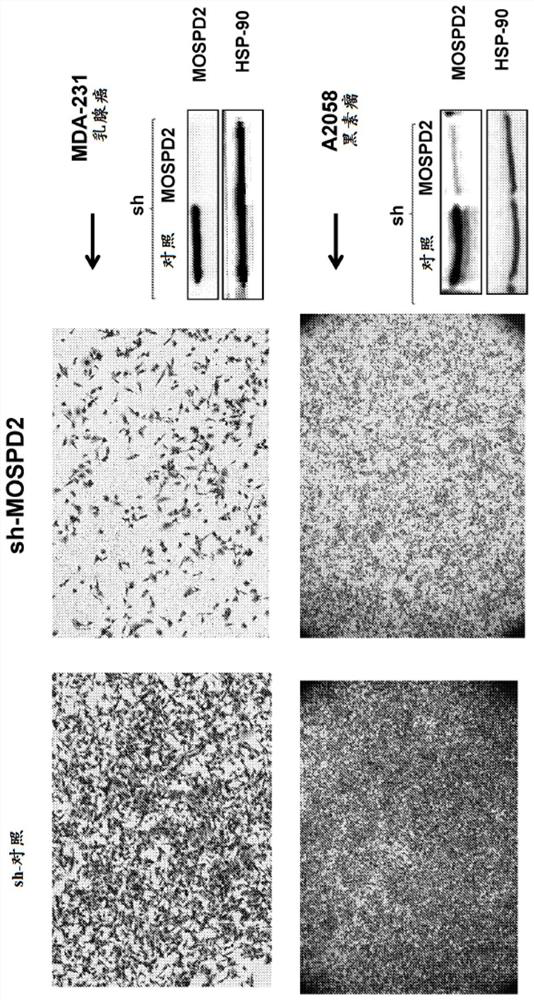
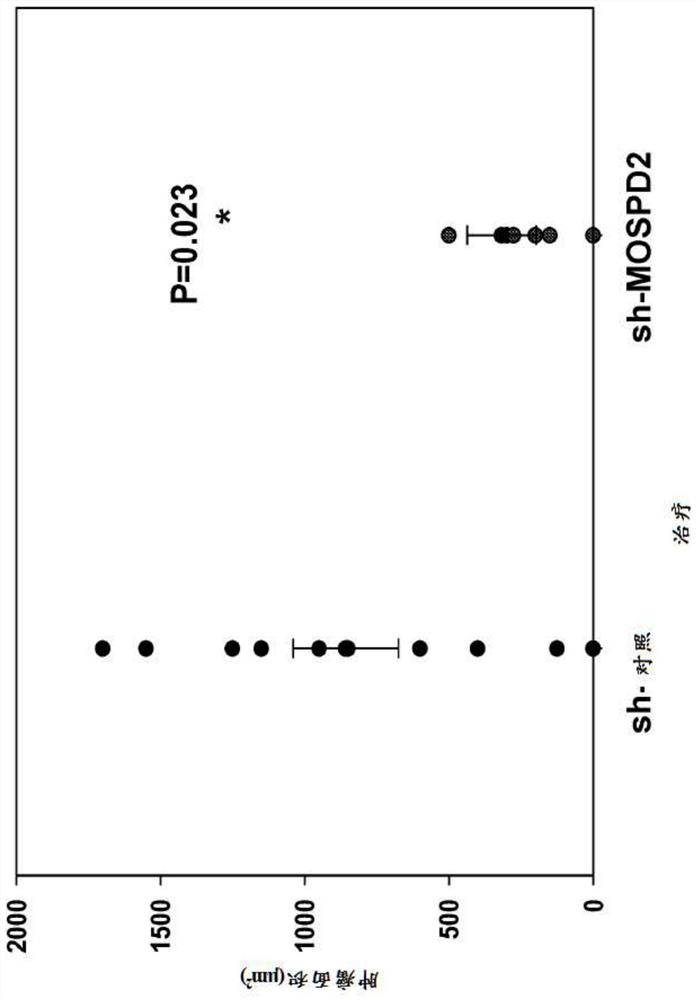
[0363] To assess the role of MOSPD2 in cancer cell migration, sh-control or sh-MOSPD2 lentiviral particles were used to silence MOSPD2 expression in two melanoma cell lines, A2058 melanoma and MDA-231 breast cancer.
[0364] Specifically, sh-control or sh-MOSPD2-transduced A2058 or MDA-231 cells (3x10 5 ) were inoculated in the upper chamber of a QCM 24-well 5 μm well migration assay plate (Corning-Costar, Corning, NY), followed by 10% FBS / RPMI-1640 and EGF (200 ng / ml, Peprotech Israel) in the lower chamber Incubate in presence for 24 hours. Cells that had migrated to the lower chamber were then stained with crystal violet before images were acquired.
[0365] figure 1 It was confirmed that sh-MOSPD2 lentiviral particles had significantly reduced protein epitopes and inhibited cell migration in vitro.
Embodiment 3
[0367] MOSPD2 and cell proliferation
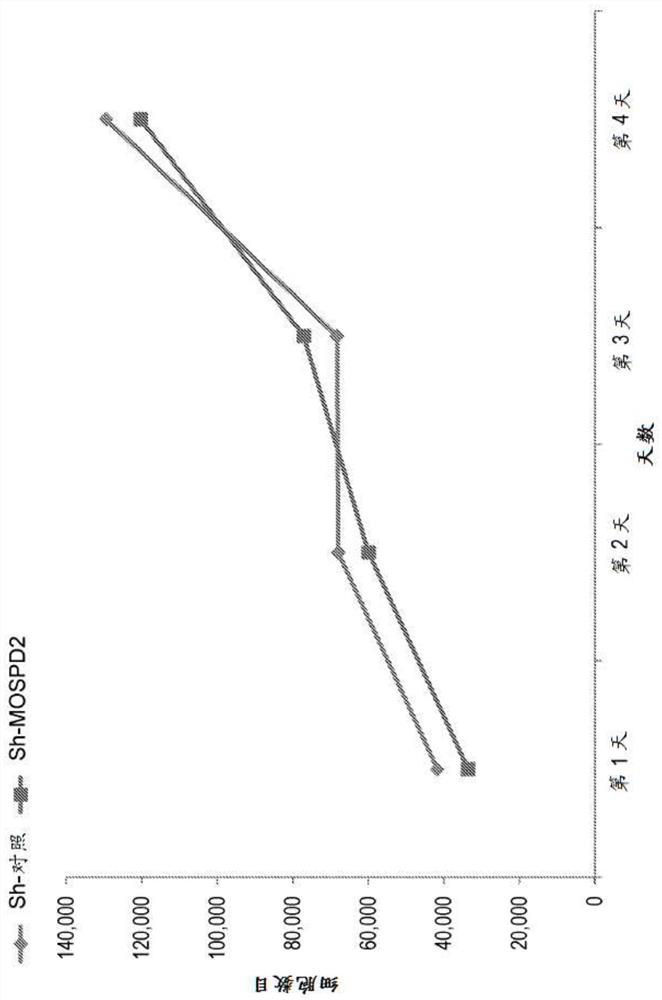
[0368] To determine whether the inhibition of cell migration following MOSPD2 silencing is secondary to fundamental cellular functions such as proliferation, the proliferation of MDA-231 breast cancer cells transduced with sh-control or sh-MOSPD2 lentiviral particles was tested for 3 days time period.
[0369] Specifically, MDA-231 cells transduced with sh-control or sh-MOSPD2 lentivirus were seeded in 6-well plates (10 4 pieces / hole). Cells were counted by FACS every 24 hours in triplicate for 3 consecutive days.
[0370] exist figure 2 The data shown in indicate that MOSPD2 is not required for the proliferation of these cells, suggesting a regulatory role for MODPD2 specifically in migration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com