Multi-target compound with anticoagulant and antiplatelet activity, preparation method and application
A multi-target and compound technology, applied in the field of biomedicine, can solve the problems of dose matching, affecting clinical antithrombotic effect, bleeding, etc., and achieve the effect of avoiding dose matching and reducing bleeding risk.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0078] One aspect of the present invention provides a preparation method of the antithrombin and platelet GPⅡb / Ⅲa receptor multi-target antagonistic compound, the method comprising the following steps: (1) adopting solid-phase synthesis method to start from the carboxyl terminal according to the polypeptide sequence Beginning to insert the corresponding protected amino acids or fragments in sequence to obtain the side chain fully protected amino acid polypeptide-Wang resin; (2) acid hydrolyzing the side chain fully protected amino acid polypeptide-Wang resin with an acid hydrolysis reagent to obtain a linear polypeptide crude product; ( 3) Cyclize the crude linear polypeptide to form a disulfide bond, and then purify it using high-pressure preparative liquid phase to obtain a polypeptide sequence.
[0079] One aspect of the present invention also provides a pharmaceutical composition, the active ingredients of which include the antithrombin and the multi-target antagonistic com...
Embodiment 1
[0091] Example 1 is used to illustrate the compound and its preparation process according to one embodiment of the present invention.
[0092] 1. Preparation of Fragment I Fmoc-Gly-Gly-Gly-Gly-OH: Weigh Fmoc-Gly-2-Cl-Trt-resin (23.5g, 16.5mmol) with a degree of substitution of 0.7mmol / g and use 2.5L 25% PIP / DMF solution to remove Fmoc protection for 25 minutes, and after filtration, the resin was washed alternately with DMF and DCM for 3 times, each time not less than 1 minute. Fmoc-Gly-OH (14.8g, 50mmol) was added, stirred and reacted at 30°C for 4 hours, and the reaction endpoint was detected by the ninhydrin method. After the reaction was completed, the resin was filtered and washed alternately with DMF and DCM three times respectively.
[0093] Repeat the above steps 2 times to connect another 2 Gly to finally obtain Fmoc-Gly-Gly-Gly-Gly-2-Cl-Trt-resin. The obtained resin was dissolved in 5 L of 30% hexafluoroisopropanol / DCM solution, stirred and reacted for 2 hours, the...
Embodiment 2
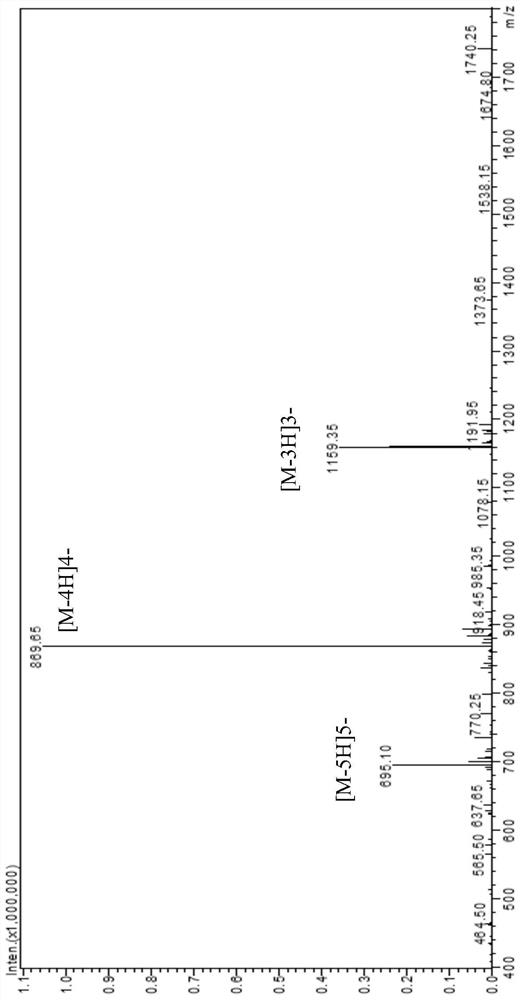
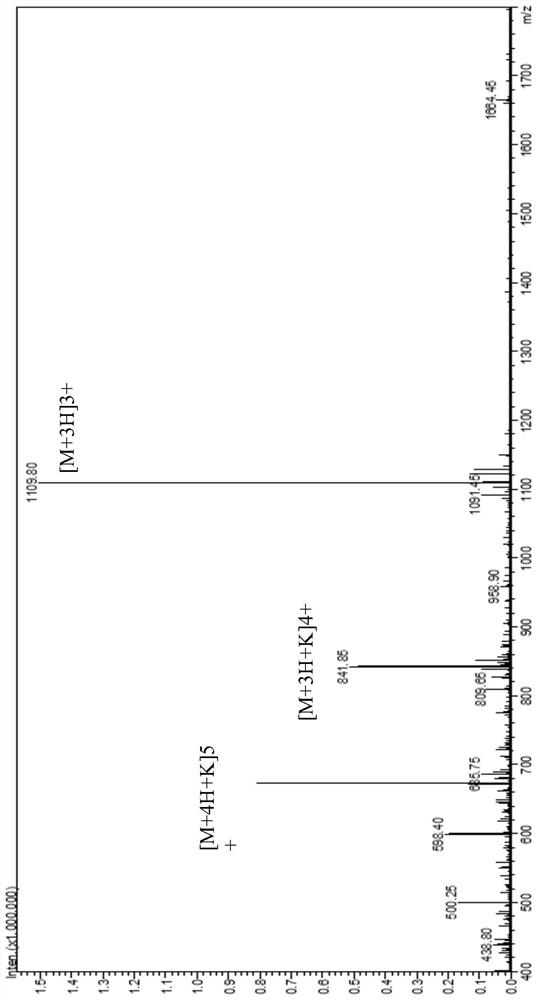
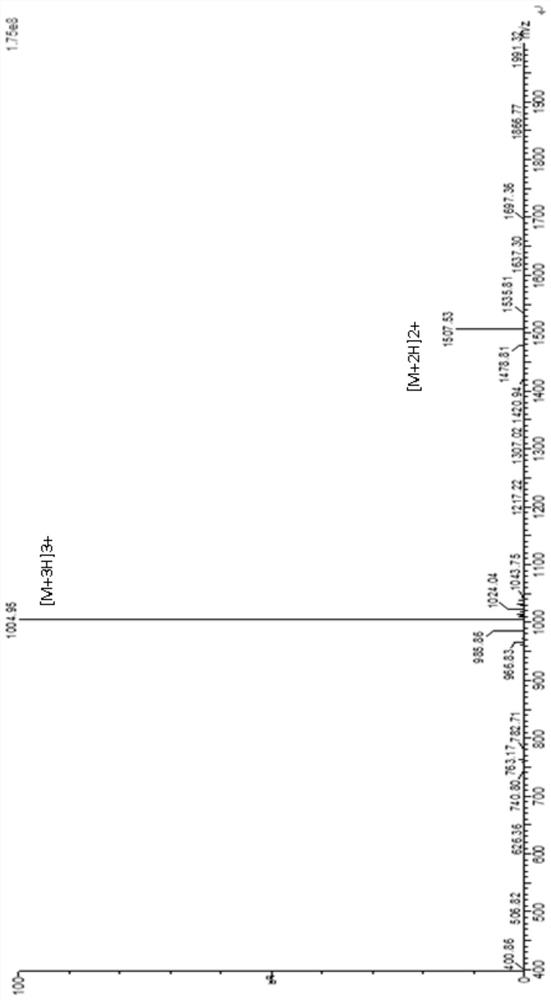
[0101] The polypeptide compound 2 was prepared according to the same method as in Example 1, except that the L' fragment was GluAlaAlaAlaLys to obtain polypeptide 2, and the product quality was 1.2 g. The mass spectrometry detection results of polypeptide 2 are as follows: figure 2shown.
[0102] The structural formula of polypeptide 2 is as follows (peptide sequence shown in SEQ ID No.5): D-Phe ProArg Pro GlyGly Gly GlyAsn GlyAsp Phe Glu Glu Ile Pro Glu Glu Tyr Leu GluAlaAlaAla LysCys Har GlyAsp Trp Pro Cys
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com