Antigen-binding molecule for promoting loss of antigens
A technology for antigen-binding molecules and antigens, applied in the fields of antibodies, anti-inflammatory agents, anti-tumor drugs, etc., can solve the problem of enhancing the binding activity of Fcγ receptors without any attempt
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[1451] Methods for the preparation of antigen-binding molecules
[1452] In addition, the present invention provides a method for producing an antigen-binding molecule comprising: an antigen-binding domain that has human FcRn-binding activity in an acidic pH range and whose antigen-binding activity varies depending on ion concentration conditions; and, in Fcγ receptor binding activity under neutral pH range Fcγ receptor binding activity higher than Fcγ receptor binding activity in which the sugar chain linked to position 297 of EU numbering is a natural type Fcγ receptor binding domain containing fucose sugar chains domain.
[1453] That is, the present invention provides a method for producing an antigen-binding molecule, the method comprising the following steps (a) to (f):
[1454] (a) a step of obtaining the antigen-binding activity of the antigen-binding domain under conditions of high calcium ion concentration;
[1455] (b) a step of obtaining the antigen-binding act...
Embodiment 1
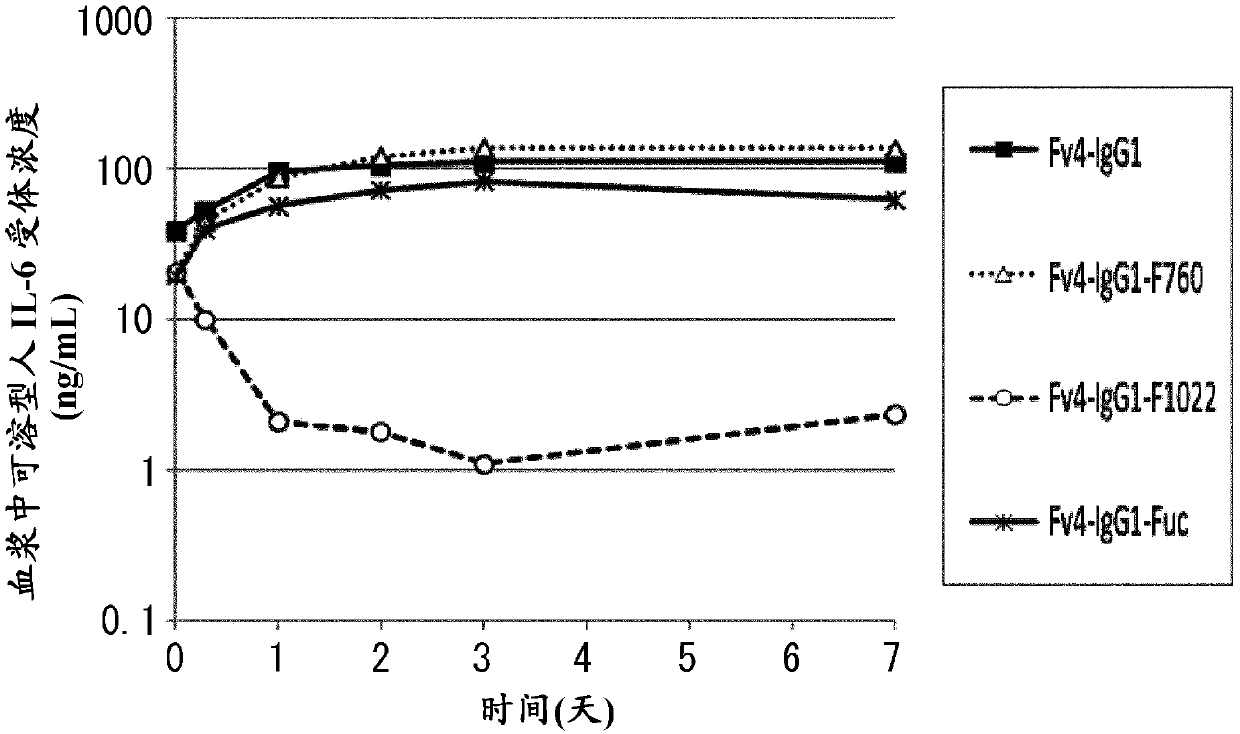
[1653] (Example 1) Production of an antigen-binding molecule having a higher binding activity to mouse FcγR than to the Fc region of native human IgG under conditions in the neutral pH range
[1654] (1-1) About pH-dependent human IL-6 receptor binding antibody
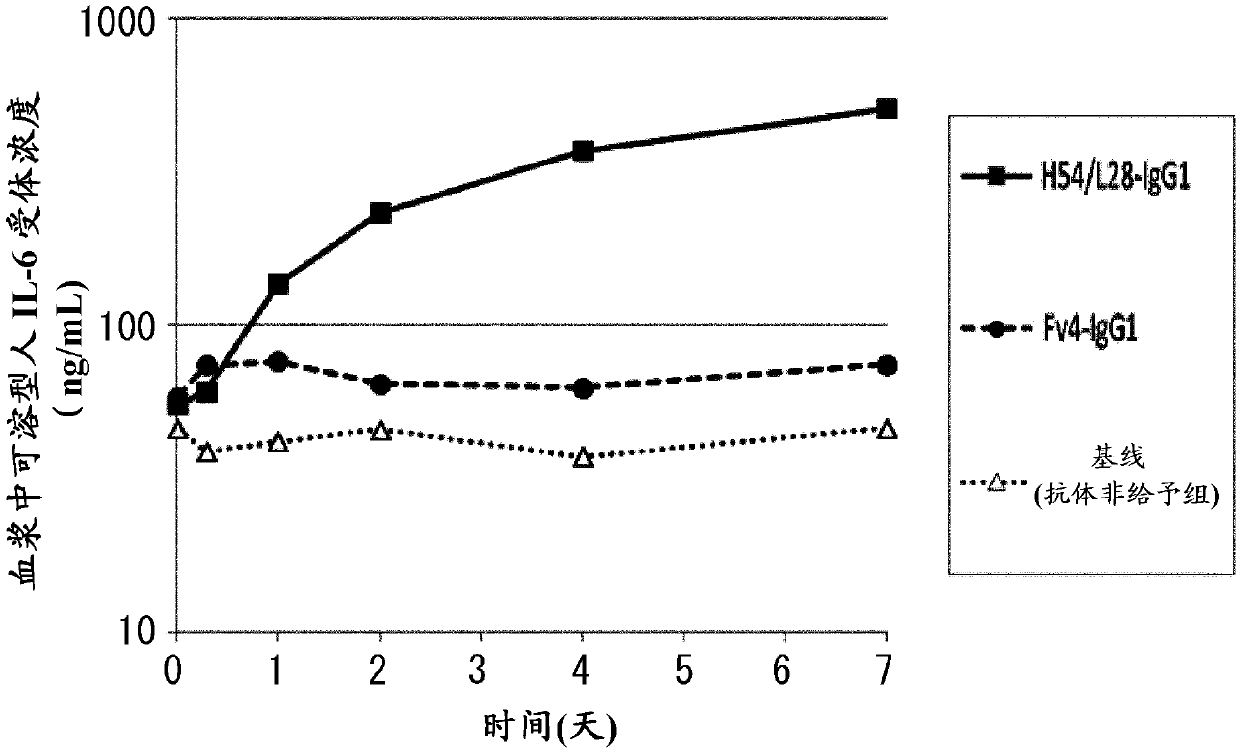
[1655] H54 / L28-IgG1 comprising H54-IgG1 (SEQ ID NO: 36) and L28-CK (SEQ ID NO: 37) described in WO2009 / 125825 is a humanized anti-IL-6 receptor antibody comprising VH3-IgG1 (SEQ ID NO: 38) and the Fv4-IgG1 of VL3-CK (SEQ ID NO: 39) is that H54 / L28-IgG1 endows the characteristic (at pH7 .4 binding, dissociation at pH 5.8) humanized anti-IL-6 receptor antibody. In the in vivo test of mice described in WO2009 / 125825, compared with the group administered with a mixture of H54 / L28-IgG1 and antigen-soluble human IL-6 receptor, when Fv4-IgG1 and antigen-soluble human IL-6 receptor were administered, In the group of mixtures of soluble human IL-6 receptors, it was shown that the elimination of soluble human IL-6 receptors...
Embodiment 2
[1669] (Example 2) Effect of Antigen-Binding Molecules with FcγR Binding Activity Higher than Fc Region Binding Activity of Natural Human IgG from Plasma
[1670] (2-1) Effect of H54 / L28-IgG1 and Fv4-IgG1 on eliminating antigen from plasma
[1671] Anti-human IL-6 receptor antibody H54 / L28-IgG1 and Fv4-IgG1 having a pH-dependent binding property to human IL-6 receptor were produced by the method of Reference Example 1. An in vivo infusion test using the prepared H54 / L28-IgG1 and Fv4-IgG1 was carried out by the following method.
[1672] (2-1-1) In vivo injection test using human FcRn transgenic mice
[1673] Subcutaneous implantation of soluble human The infusion pump of IL-6 receptor (MINI-OSMOTIC PUMP MODEL2004, alzet) was used to create an animal model in which the concentration of soluble human IL-6 receptor in plasma was maintained at a steady state. Anti-human IL-6 receptor antibody was administered to this animal model, and the in vivo kinetics after administrati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com