Zika virus and encephalitis B virus combined inactivated vaccine
A technology for Japanese encephalitis virus and Zika virus, applied in the field of combined inactivated vaccine, can solve the problem of no joint inactivated vaccine development of Japanese encephalitis virus and Zika virus, so as to reduce the number of vaccinations, cause good disease, prevent and control disease-causing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The cultivation technique of embodiment 1 Japanese encephalitis virus vaccine
[0030] After the Vero cells are cultured to a single layer on the microcarrier, the cell culture medium is discarded, the cell surface is washed with PBS, and the JE virus is inoculated, and the second-generation working virus of the JE rat brain virus strain P3 is used to pass on the Vero cells. species, the inoculation MOI is 0.01CCID 50 / ml, the pH of the virus culture medium was 7.6, and it was cultured at 35°C. The supernatant was harvested for the first time 2 days after inoculation with JE virus, and the harvested volume was 80% of the total liquid volume. Afterwards, the supernatant was harvested every two days according to the aforementioned instructions, for a total of 5 harvests. The harvested supernatants of each batch were combined to obtain the Japanese encephalitis virus harvesting liquid.
Embodiment 2
[0031] The purification of embodiment 2 Japanese encephalitis virus
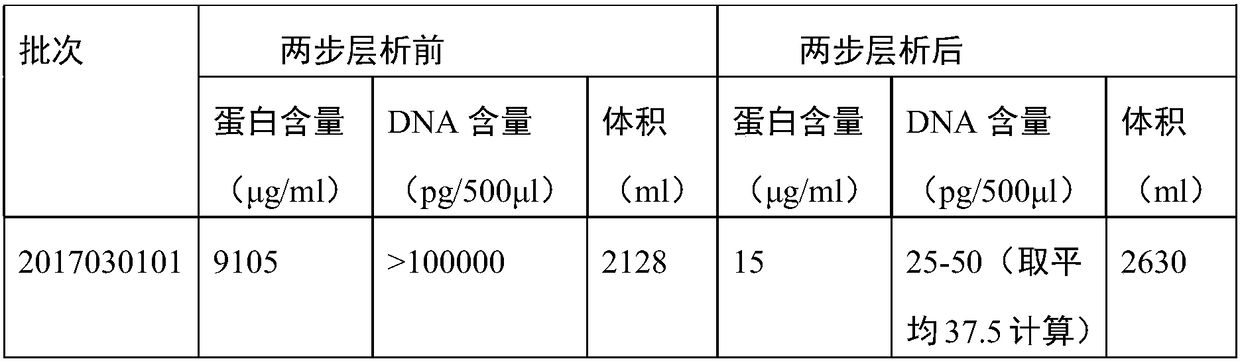
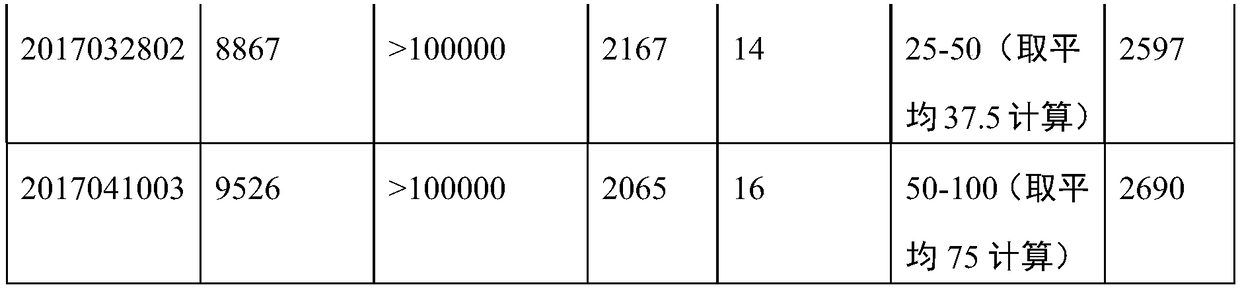
[0032] After the JE virus harvest solution was filtered through a deep membrane stack to remove cell debris and large clumps, the cell supernatant was harvested. Concentrate by ultrafiltration with a molecular weight cut-off of 300,000 to obtain a concentrate. After gel filtration chromatography and ion exchange chromatography.
[0033] Among them, gel filtration chromatography can use GE Sepharose 4Fast Flow, Sepharose 4FastFlow, Sephacryl S-300HR, Sephacryl S-400HR, Sephacryl S-500HR and other high-throughput and high-capacity fillers with suitable separation range and easy scale-up. The optional fillers for ion exchange chromatography are GE DEAE, CM and ANX fillers. The specific operation is as follows.
[0034] (1) At first the Japanese encephalitis virus vaccine concentrate is filtered through the chromatographic column, the ultraviolet detection wavelength is 280nm, the flow velocity is 6cm / h, and ...
Embodiment 3
[0042] The inactivation process of embodiment 3 Japanese encephalitis virus
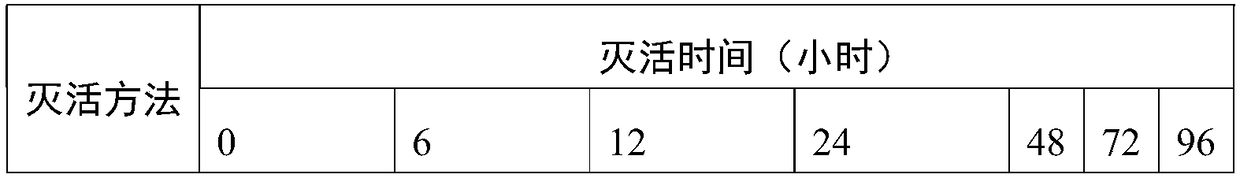
[0043] The Japanese encephalitis virus purification solution prepared in Example 2 was inactivated, and inactivated according to two inactivation methods, respectively, β propiolactone with a final concentration of 0.025% was inactivated at 4°C and the final concentration was 30-180ug / ml formaldehyde 25 Inactivation at ±3°C, samples were taken at 0, 6, 12, 24, 36, 48, 72, and 96 hours, and the virus titer was detected. The results were analyzed using SPSS17.0 software, expressed as (x±s), according to Analysis of variance and t-test were used, and P<0.05 was considered statistically significant. The results are shown in Table 2. After 6 hours of formaldehyde inactivation, the virus titer began to decline. After 24 hours, most of the viruses had been inactivated, and no virus titer was detected after 48 hours. No virus can be detected after 12 hours of β-propiolactone inactivation.
[0044] Samples ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com