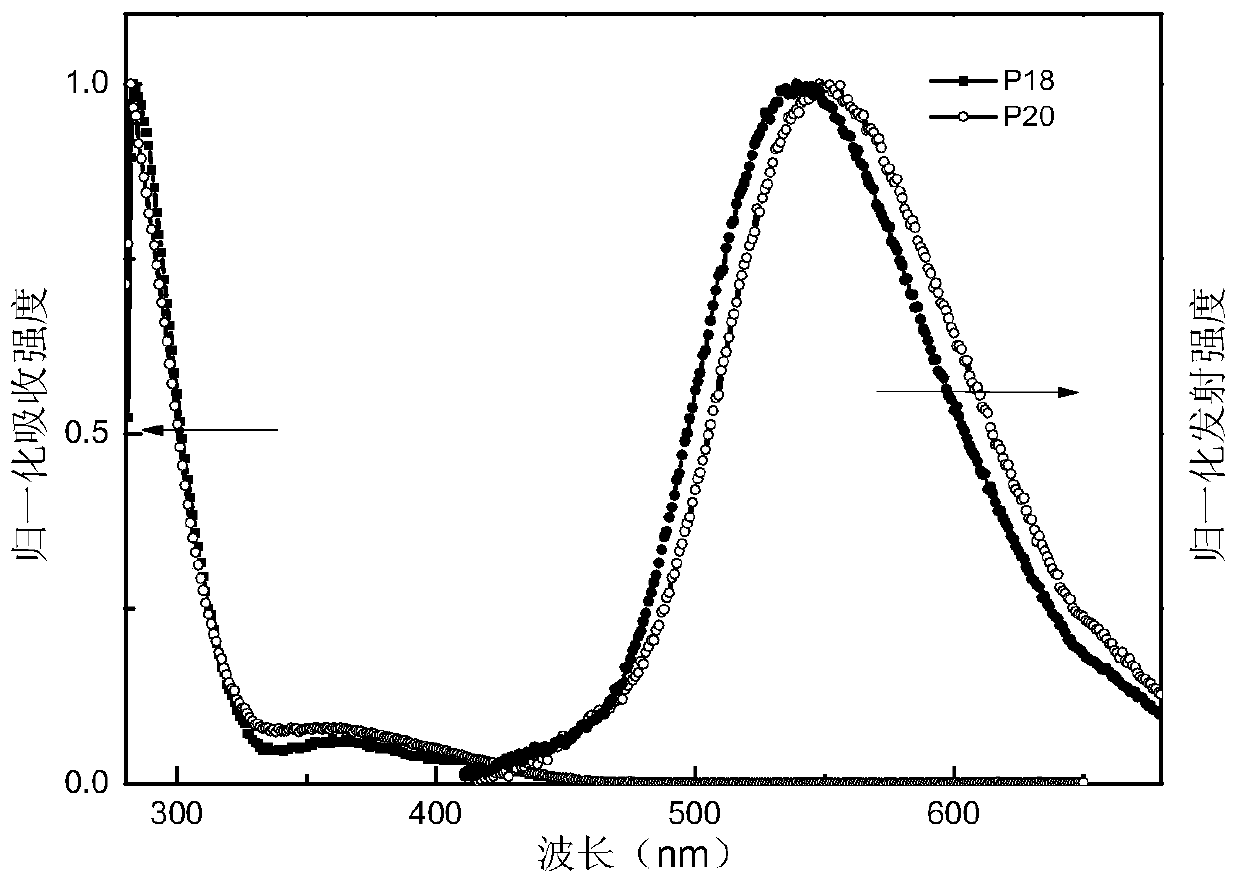
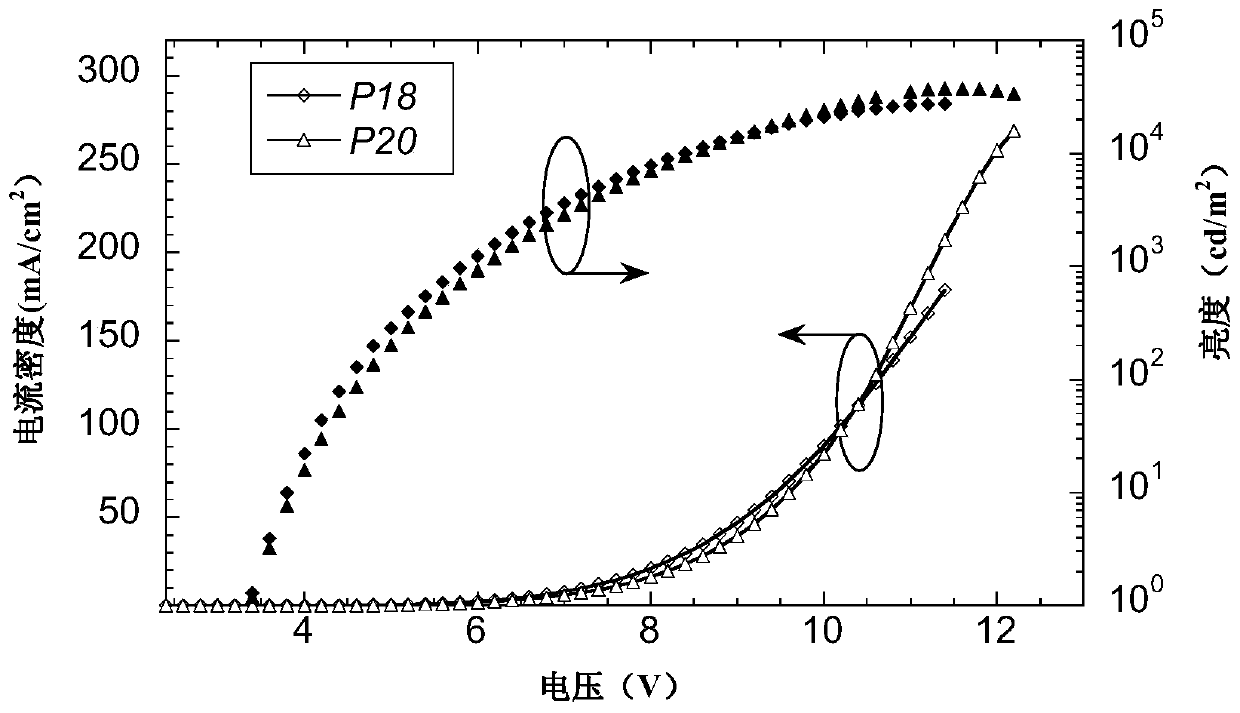
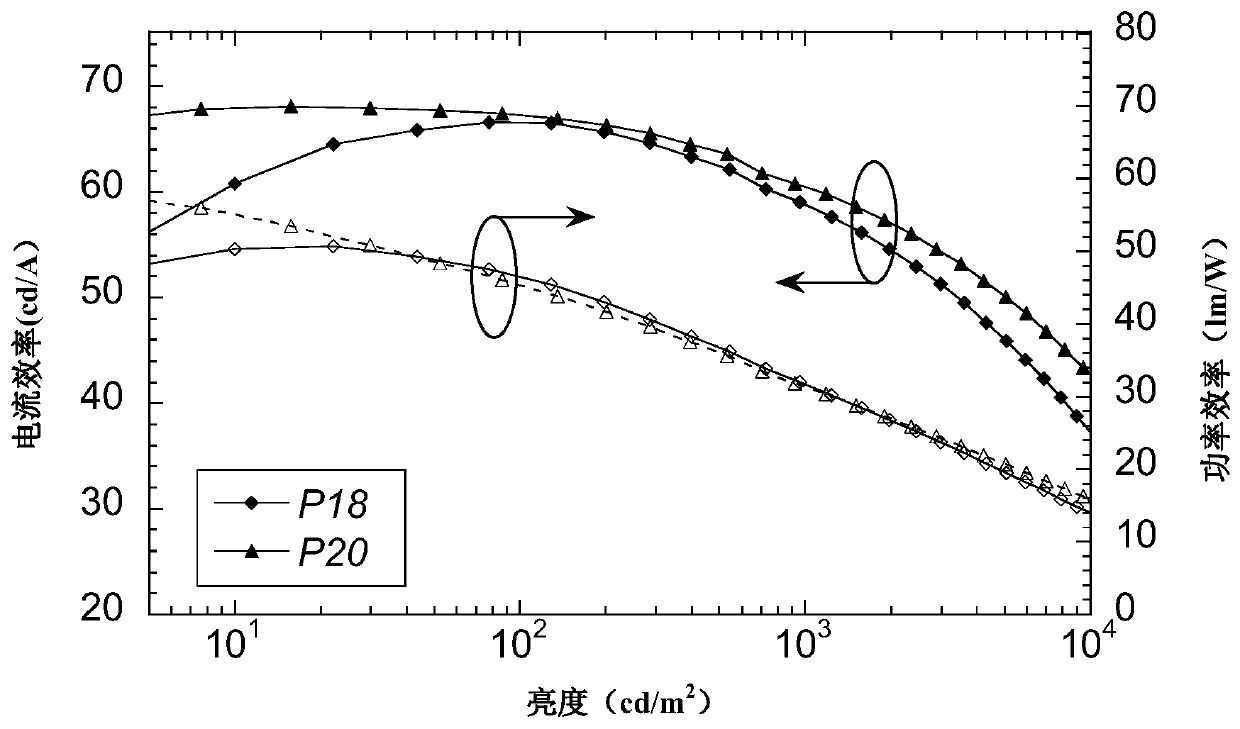
A kind of double acceptor organic light-emitting small molecule material and its preparation method and application
A small molecule, double acceptor technology, applied in the preparation of light-emitting materials, organic compounds, organic chemistry, etc., can solve the problem of low utilization of excitons, achieve the effect of determining molecular weight, simplifying device structure, and improving device performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This embodiment prepares intermediates 1 to 14 and compound P1:
[0044] The preparation steps of intermediate 1 are:
[0045]
[0046] Under a nitrogen atmosphere, 11.7g (40.0mmol) of p-bromoiodobenzene, 5.8g (24.0mmol) of sodium sulfide nonahydrate, 336mg (0.1equ) of cuprous iodide (CuI), anhydrous carbonic acid Potassium 5.4g (40.0mmol), N,N-dimethylformamide (DMF) 80mL. Heated to 120°C, and stirred for 18 hours in the dark. After the reaction, the solvent in the reaction system was suspended to dryness, extracted three times with dichloromethane and water, and the organic phase was taken. Dichloromethane was distilled off under reduced pressure, and purified by a silica gel column to obtain 7 g of the intermediate of structural formula 1 with a yield of 94%, C12H8Br2S M / Z=341.87. m / z: 343.87 (100.0%), 341.87 (51.4%), 345.87 (48.6%), 344.87 (9.7%), 346.87 (6.3%), 345.87 (4.5%), 342.87 (4.4%), 344.87 (3.2%) ), 343.87 (2.3%), 342.87 (2.2%), 347.86 (2.2%); elemen...
Embodiment 2
[0089] This embodiment prepares compound P2, and its structural formula and synthetic route are as follows:
[0090]
[0091] The specific implementation steps are:
[0092] Intermediate 14 in Example 1 was replaced with an equivalent amount of Intermediate 10, and other raw materials and steps were the same as in Example 1 to obtain 1.50 g of the product of structural formula P2, with a yield of 76%. Molecular formula: C43H32N2O3S; M / Z=656.80 Theoretical value: m / z: 656.21 (100.0%), 657.22 (46.5%), 658.22 (10.6%), 658.21 (4.5%), 659.21 (2.1%); Elemental analysis: C , 78.63; H, 4.91; N, 4.27; O, 7.31; S, 4.88.
Embodiment 3
[0094] This embodiment prepares compound P3, and its structural formula and synthetic route are as follows:
[0095]
[0096] The specific implementation steps are:
[0097] The intermediate 14 in the preparation step of P1 in Example 1 was replaced with an equivalent amount of Intermediate 12, and other raw materials and steps were the same as in Example 1 to obtain 1.45 g of the product of structural formula P3, with a yield of 74%. Molecular formula: C43H32N2O3S; M / Z=656.80 Theoretical value: m / z: 656.21 (100.0%), 657.22 (46.5%), 658.22 (10.6%), 658.21 (4.5%), 659.21 (2.1%); Elemental analysis: C , 78.63; H, 4.91; N, 4.27; O, 7.31; S, 4.88.
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission peak | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com