Application of Anti-egfr scFv::FTH1/FTH1 Protein Nanoparticles in Drug Preparation
A nanoparticle and protein technology, applied in the field of nanobiomedicine, can solve the problems of unclear anti-EGFR antibody effect and unknown pathogenesis, and achieve the effect of reducing the number of goblet cells, expanding the cross-sectional area, and reducing airway resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
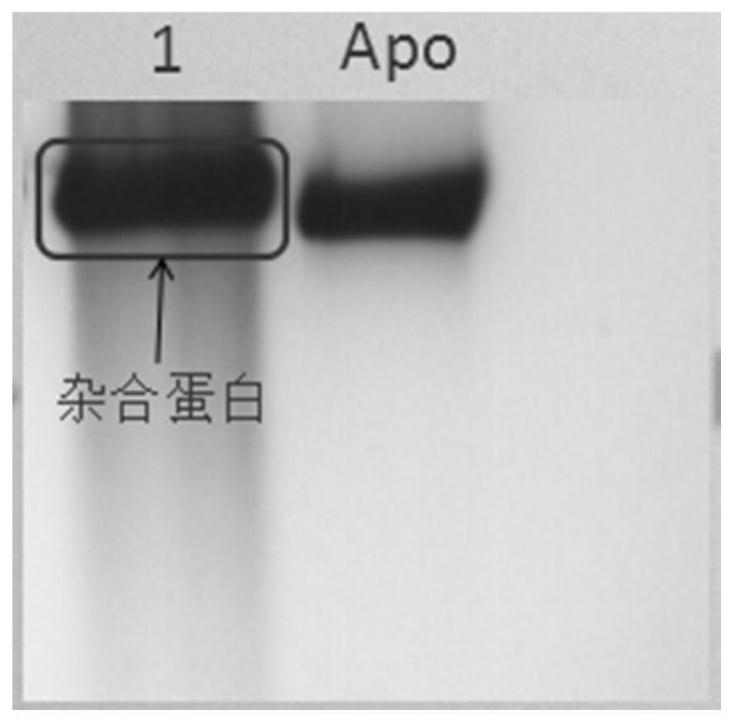
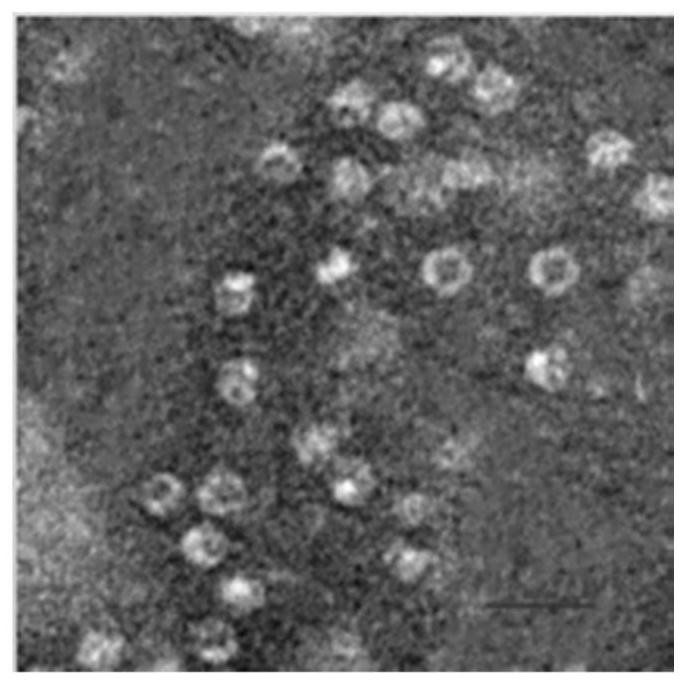
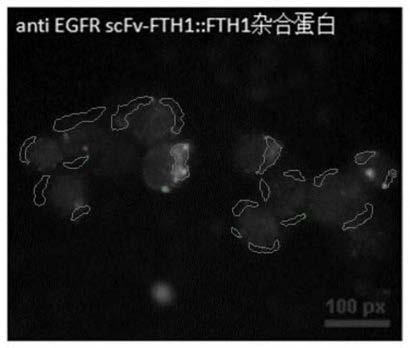
[0026] Example 1 Preparation and characterization of anti-EGFR scFv::FTH1 / FTH1 protein nanoparticles
[0027] Two plasmids pET-28(+)-FTH1, pET-28a(+)-anti-EGFR scFv-FTH1 exist in our laboratory (see ZL201010239499.3 for specific preparation methods). Wherein, the amino acid sequence of the anti-EGFR scFv::FTH1 protein is shown in SEQ ID NO. 1, and the amino acid sequence of the FTH1 protein is shown in SEQ ID NO.2. Escherichia coli competent cells E.coli.BL21(DE3) and E.coli.DH5α were purchased from Beijing Tiangen Biochemical Technology Company. The above two plasmids were transformed into E. coli. DH5α competent cells, and positive clones were screened by colony PCR and restriction enzyme identification. After that, the expression vector with the correct sequence was transformed into Ecoil.BL21(DE3) competent cells by sequencing to obtain the target engineering expression strain cells containing the FTH1 protein gene and the anti-EGFR scFv::FTH1 protein gene target engineering...
Embodiment 2
[0033] Example 2 The effect of anti-EGFR scFv::FTH1 / FTH1 protein nanoparticles on the number of goblet cells and bronchial mucus secretion in mouse lungs
[0034] 1. Experimental materials
[0035] 1.1 Subject
[0036] SPF BALB / c mice, weighing 18-20 grams and 6-8 weeks old, were randomly divided into 2 groups.
[0037] 1.2 Experimental reagents
[0038] 1) Intraperitoneal injection of sensitizing solution, which contains ovalbumin (OVA) and Al(OH) 3 PBS solution, OVA concentration is 10mg / L, 0.2ml per mouse is injected intraperitoneally.
[0039] 2) The excitation solution is a PBS solution with an OVA concentration of 20 mg / L, and the excitation method is atomization excitation. The average median diameter is 2.9 μm atomized particles, and the atomization time is 30 minutes.
[0040] 3) All other reagents are prepared with sterile PBS for analytically pure drugs.
[0041] 4) Anti-EGFR scFv::FTH1 / FTH1 protein nanoparticles, prepared in Example 1.
[0042] 2. Experimental method
[0043] Ast...
Embodiment 3
[0052] Example 3 The effect of anti-EGFR scFv::FTH1 / FTH1 protein nanoparticles on the deposition of collagen fibers under the bronchial epithelium of mice
[0053] 1. Experimental method
[0054] 1) Asthma model construction is the same as in Example 2, that is, after the first, third, and seventh times of nebulization, PBS and anti-EGFR scFv::FTH1 / FTH1 protein nanoparticles are injected intraperitoneally into mice respectively. The mice were dissected 24 hours after the last injection, and lung tissues of the mice were collected. Section staining detects the deposition of collagen fibers under the bronchial epithelium, which is an important indicator of airway remodeling in bronchial asthma.
[0055] 2) The experimental group design, paraffin section technique and pre-treatment of section staining are the same as in Example 2.
[0056] 3) The processed tissue sections were stained with MASSON trichrome. After the paraffin sections were deparaffinized to water, they were washed with ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com