Use of dihydroisoquinolinium double derivatives for treating keratin materials, compositions and implementation processes
A technology of keratin fibers and uses, applied in the field of human keratin fibers such as hair, medium compositions, compositions for treating keratin materials, and compositions for brightening keratin materials, which can solve problems such as skin irritation and achieve brightening improvement Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 approach
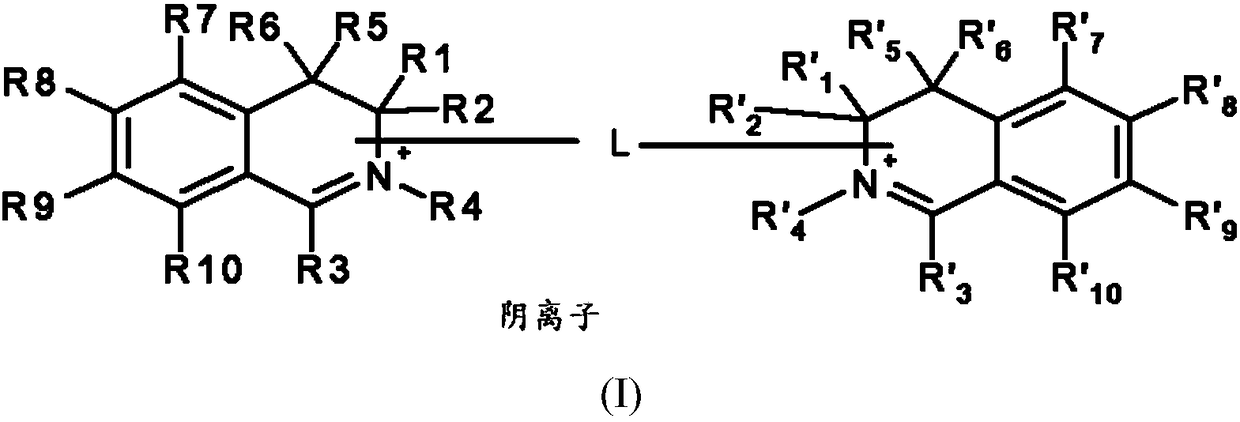
[0159] ●R 1 and R' 1 same as each other, R 2 and R' 2 same as each other, R 3 and R' 3 same as each other, R 5 and R' 5 same as each other, R 6 and R' 6 same as each other, R 7 and R' 7 same as each other, R 8 and R' 8 same as each other, R 9 and R' 9 same as each other, R 10 and R' 10 are identical to each other, and each pair independently represents a group selected from the following:
[0160] -A hydrogen atom;
[0161] - a halogen atom,
[0162] - C optionally substituted by one or more groups 1 -C 6 Alkyl, the one or more groups may be the same or different, selected from hydroxyl, cyano, C 1 -C 6 Alkoxy and amino NR a R b group,
[0163] -C 1 -C 6 alkoxy;
[0164] - Hydroxy;
[0165] - Amino NR a R b ,
[0166] -Aminocarbonyl –CONH 2 ,
[0167] -Carboxyl –CO 2 H,
[0168] ●R 4 and R' 4 together form a divalent group L such that L represents a linear or branched, saturated or unsaturated C 1 -C 15 Alkylene, optionally substituted by...
no. 2 approach
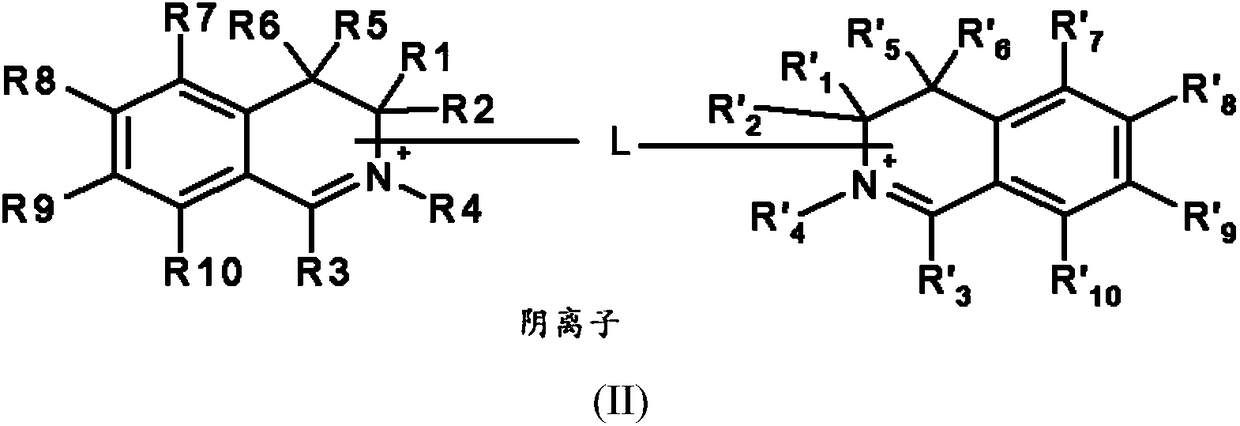
[0192] ●R 1 and R' 1 same as each other, R 2 and R' 2 same as each other, R 5 and R' 5 same as each other, R 6 and R' 6 same as each other, R 7 and R' 7 same as each other, R 8 and R' 8 same as each other, R 9 and R' 9 same as each other, R 10 and R' 10 are identical to each other, and each pair independently represents a group selected from the group consisting of:
[0193] -A hydrogen atom;
[0194] - a halogen atom,
[0195] - C optionally substituted by one or more groups 1 -C 6 Alkyl, the one or more groups may be the same or different, selected from hydroxyl, cyano, C 1 -C 6 Alkoxy and amino NR a R b group,
[0196] -C 1 -C 6 alkoxy;
[0197] - Hydroxy;
[0198] - Amino NR a R b ,
[0199] -Aminocarbonyl –CONH 2 ,
[0200] -Carboxyl –CO 2 H,
[0201] ●R 4 and R' 4 are the same and represent C optionally substituted with one or more groups 1 -C 6 Alkyl, the one or more groups may be the same or different, selected from hydroxyl, C 1 -...
Embodiment 1
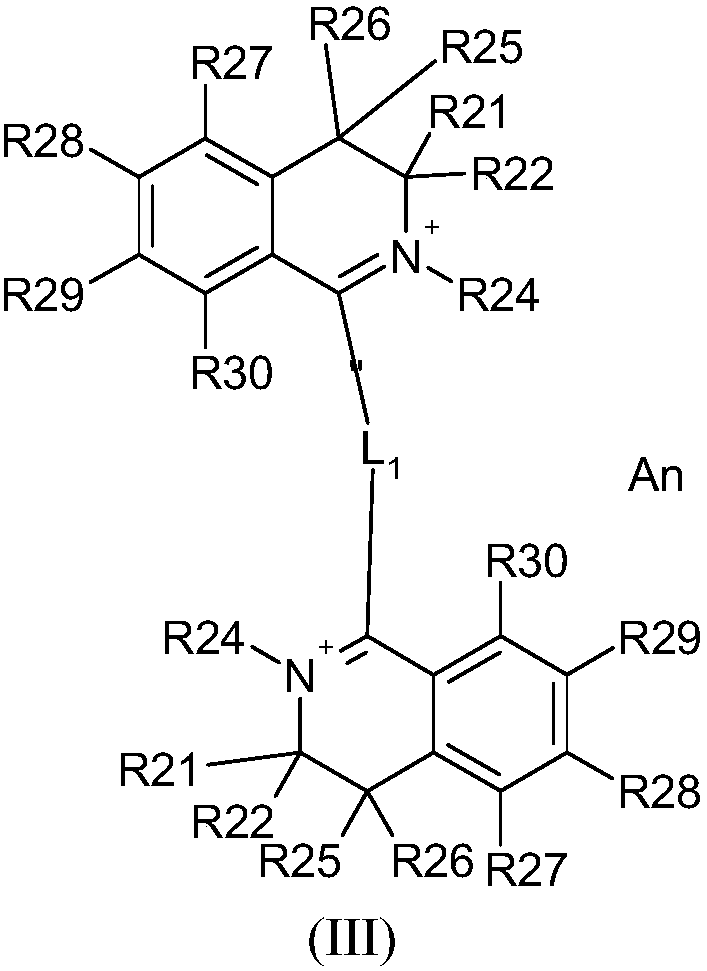
[0392] Synthesis of 2,2'-butane-1,4-diyldi-3,4-dihydroisoquinolinium dibromide (compound 1)
[0393]
[0394] 10 g of 3,4-dihydroisoquinoline (4 equivalents) was placed in 75 mL of toluene in a 250 mL three-necked flask, followed by the addition of 2.28 mL of 1,4-dibromobutane (1 equivalent). The mixture was refluxed for 15 hours with vigorous stirring. The beige-brown precipitate was filtered off under vacuum and under argon, washed with diisopropyl ether, and then washed with P 2 o 5 dry.
[0395] The product was recrystallized from 80 mL of n-propanol and isolated as a beige colored powder (5.15 g, yield = 56.5%).
[0396] Analysis is based on expected product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com