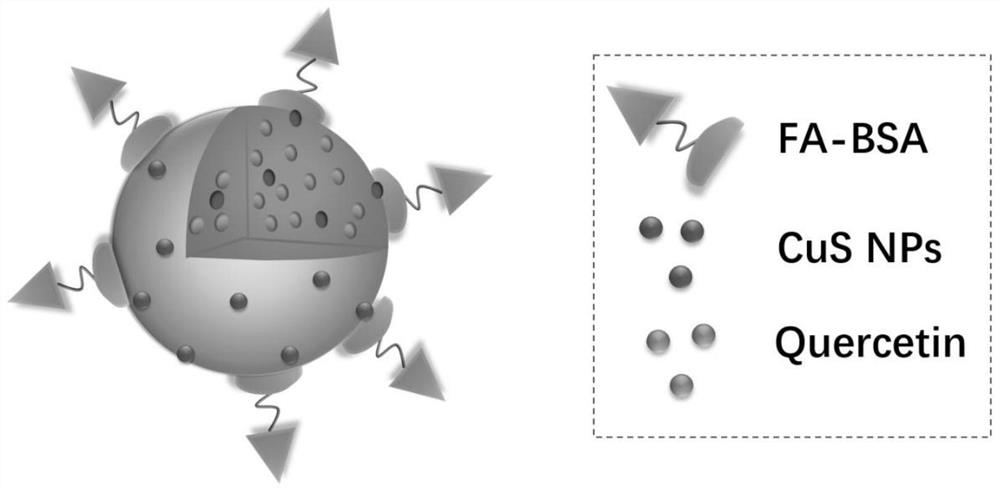
A quercetin drug delivery system based on copper sulfide-metal organic framework materials
A drug-loading system, quercetin technology, applied in the field of medicine, to achieve the effects of increasing sustained release, improving chemical stability, and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Example 1 Preparation of FA-BSA / CuS@ZIF-8-QT
[0049] 1) A certain amount of PVP is dissolved in water, and copper chloride pentahydrate is added. After stirring at room temperature for 30 minutes, sodium sulfide was added, followed by reaction in an oil bath at 90°C for 15 minutes. After cooling to room temperature, CuS NPs were collected by centrifugation and dispersed in methanol. 2-Methylimidazole was dissolved in methanol, mixed with the above-mentioned CuS methanol solution, after stirring for a period of time, zinc nitrate was added, and the stirring was continued for 1 hour. The gray-black precipitate was collected by centrifugation and dispersed into methanol. Quercetin was dissolved in methanol, mixed with a certain amount of CuS@ZIF-8 dispersion and stirred at room temperature for one day. CuS@ZIF-8-QT was collected by centrifugation, washed twice with methanol, then twice with deionized water, and finally freeze-dried to obtain a yellow-brown powder.
[0...
Embodiment 2
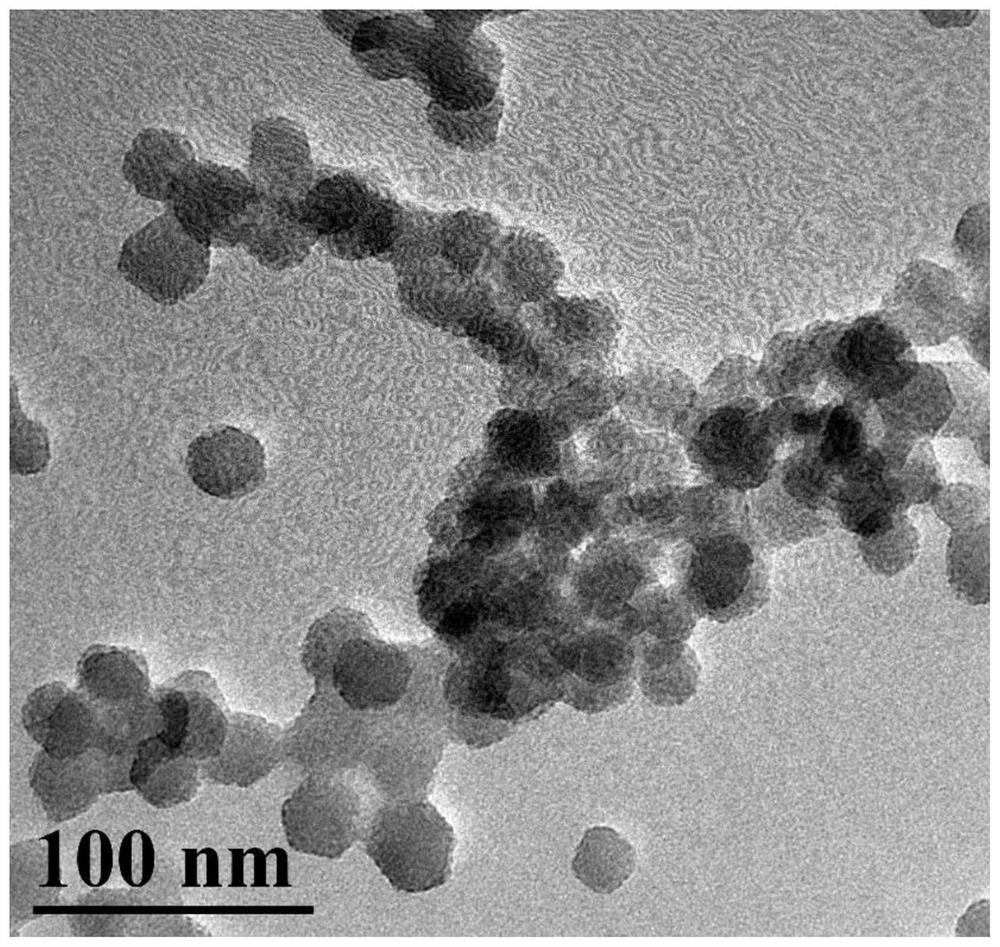
[0051] Example 2 Morphological observation of FA-BSA / CuS@ZIF-8-QT nanoaggregates
[0052] Take about 20 μL of CuS@ZIF-8 and FA-BSA / CuS@ZIF-8-QT methanol suspension respectively, drop them on the carbon-coated copper mesh, absorb the excess liquid with filter paper, dry them under infrared light and place them under the transmission electron microscope Observe the morphology of FA-BSA / CuS@ZIF-8-QT nanoaggregate. Electron microscope photos such as figure 2 As shown, FA-BSA / CuS@ZIF-8-QT is a spherical shape with uniform particle size, good dispersion, and a particle size of about 40nm, which meets the particle size requirements for intravenous injection and passive targeting.
Embodiment 3
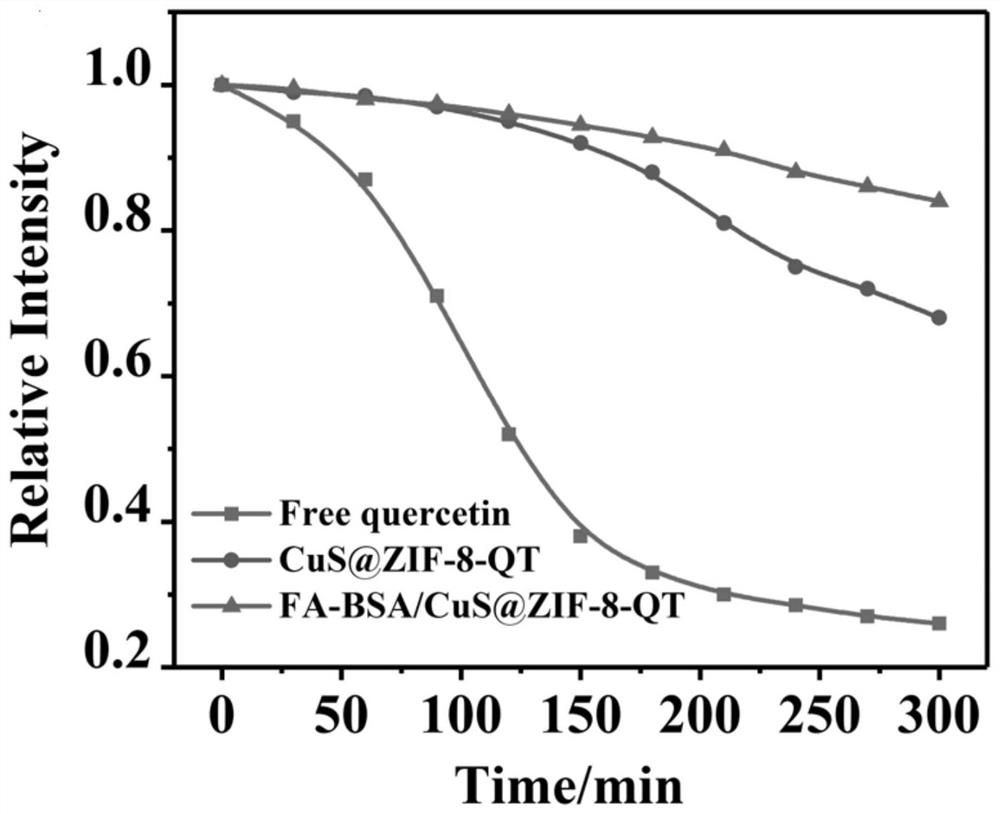
[0053] Embodiment 3 quercetin stability change research
[0054] Accurately weigh a certain amount of quercetin with an analytical balance, dilute with distilled water to prepare a suspension of 500 μg / mL, take a certain amount of CuS@ZIF-8-QT and FA-BSA / CuS@ZIF-8-QT, and configure suspension in distilled water with the same concentration of quercetin. Then take 1 mL of the above suspension, add 0.5 mL of 0.1 M hydrochloric acid, and dissolve with 8.5 mL of methanol. Measure their UV absorbance. In the following 5 hours, repeat the above operation every half hour, measure and record the corresponding ultraviolet absorption. The changes in the relative intensity of ultraviolet absorption in each group are as follows: image 3 shown. The results showed that after dispersing in water for 5 h, the relative UV absorption intensity of free quercetin decreased by about 75%, indicating that it decomposed rapidly; while the relative UV absorption of CuS@ZIF-8-QT NP group decreased ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com