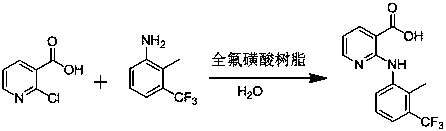
A kind of synthetic method of flunixin
A synthesis method and flunixin technology, applied in the field of chemical synthesis, can solve the problems of increasing production cost, low yield, low ethylene glycol efficiency, etc., and achieve the guarantee of product yield and quality, shorten reaction time, and reduce production. cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Add 300L water into a 1000L reactor, add 131.9kg (837.5mol) 2-chloronicotinic acid and 140kg 2-methyl-3-trifluoromethylaniline (800mol) respectively, stir, add 1.4kg perfluorosulfonic acid resin, and heat up After reacting for 2.5 hours at 65°C-70°C, filter with suction, adjust the pH of the filtrate to 4.5 with 60% sulfuric acid solution, stir for 15-20min, shake off the filter, wash the filter cake with 30kg of water, and dry the filter cake to obtain 230.1kg of flunixin , the yield is 97.1%, the purity is 99.9%, and the purity is detected according to the method in USP38, the same below.
Embodiment 2
[0036] Add 300L water into a 1000L reactor, add 131.9kg (837.5mol) 2-chloronicotinic acid and 140kg (800mol) 2-methyl-3-trifluoromethylaniline respectively, stir, add 1.4kg perfluorosulfonic acid resin, Raise the temperature to 70°C, react for 2.5 hours, then filter with suction, adjust the pH of the filtrate to 4.5 with 60% sulfuric acid solution, stir for 15-20 minutes, shake off the filter, wash the filter cake with 30 kg of water, and dry the filter cake to obtain 229.8 kg of flunixin. The yield is 97.0%, and the purity is 99.8%.
Embodiment 3
[0038] Add 300L water into a 1000L reactor, add 131.9kg (837.5mol) 2-chloronicotinic acid and 140kg (800mol) 2-methyl-3-trifluoromethylaniline respectively, stir, add 1.4kg recycled perfluorosulfonic acid resin , raised the temperature to 65°C, reacted for 2.5 hours, then filtered with suction, adjusted the pH of the filtrate to 4.5 with 60% sulfuric acid solution, stirred for 15-20 minutes, and filtered with a shaker, washed the filter cake with 30 kg of water, and dried the filter cake to obtain 229.6 kg of flunixin. Yield 96.9%, purity 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com