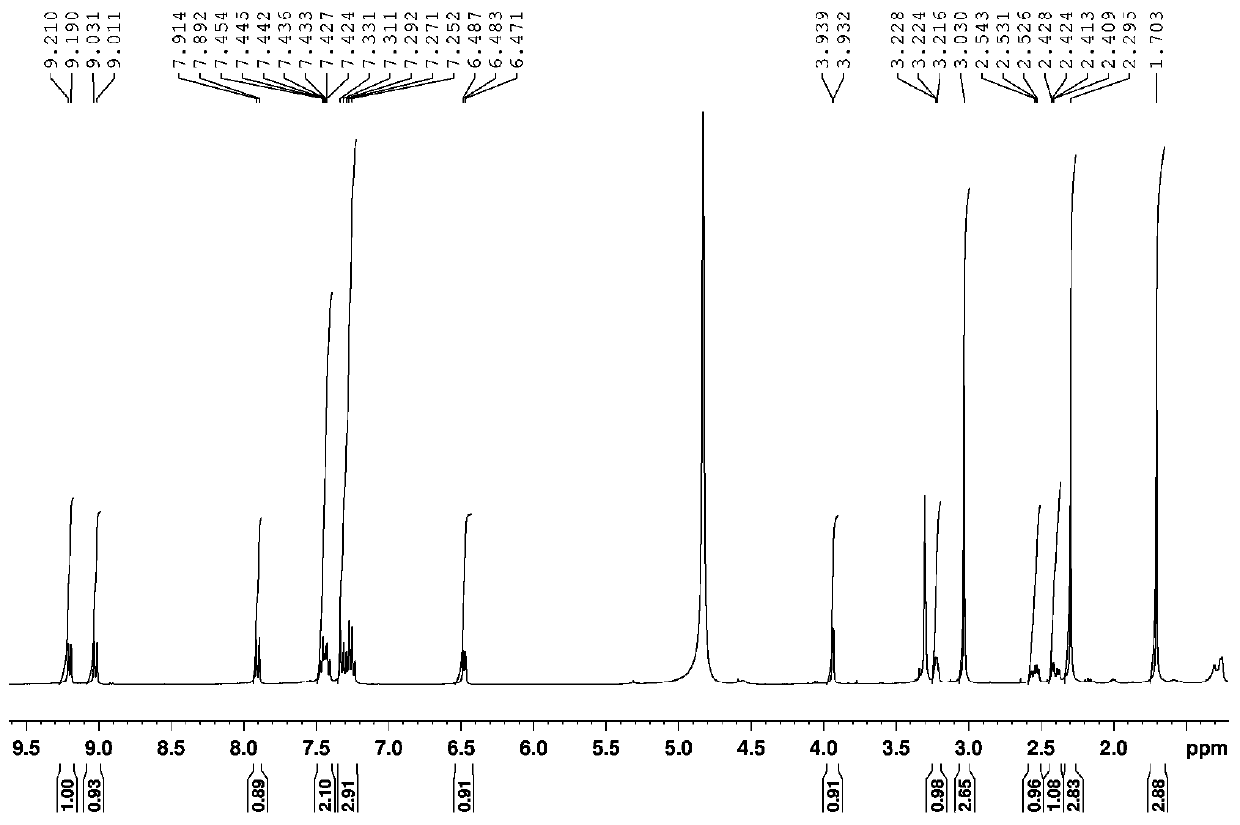
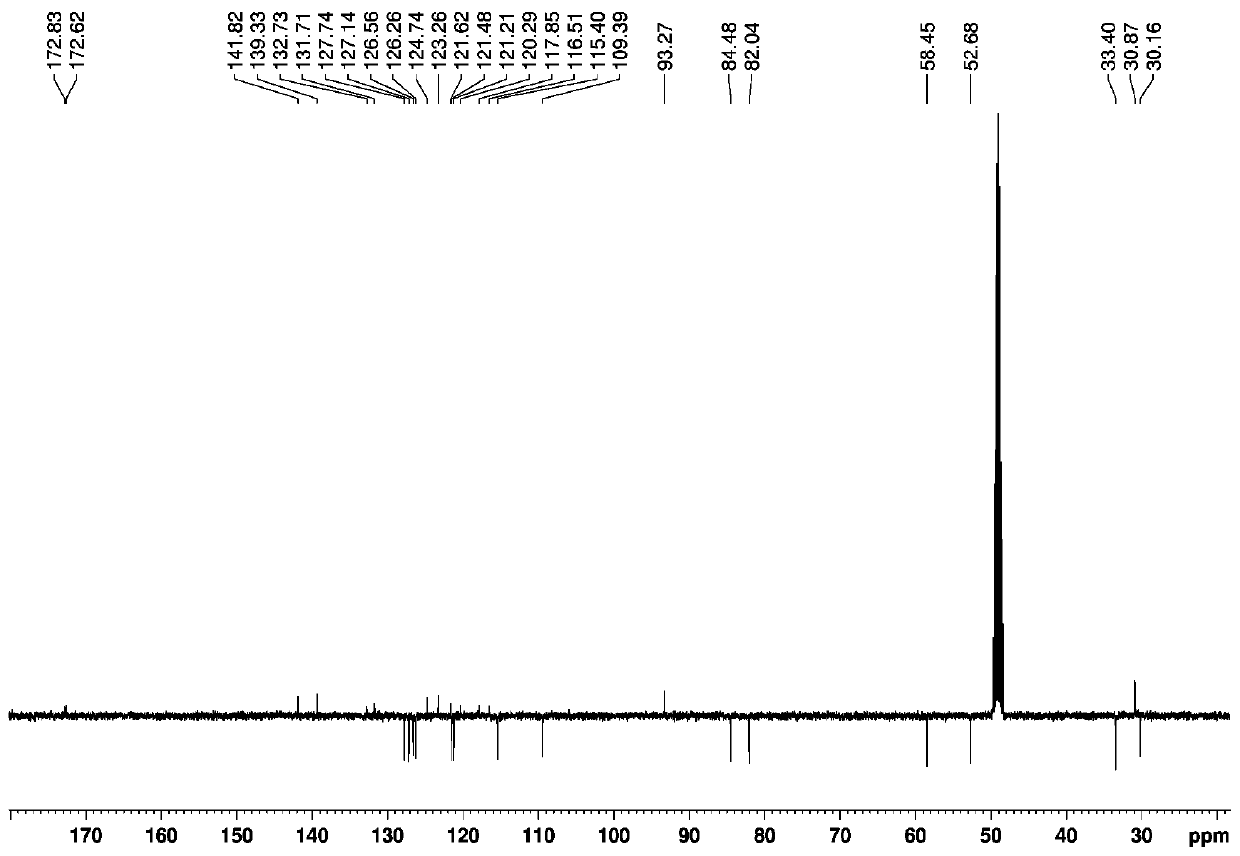
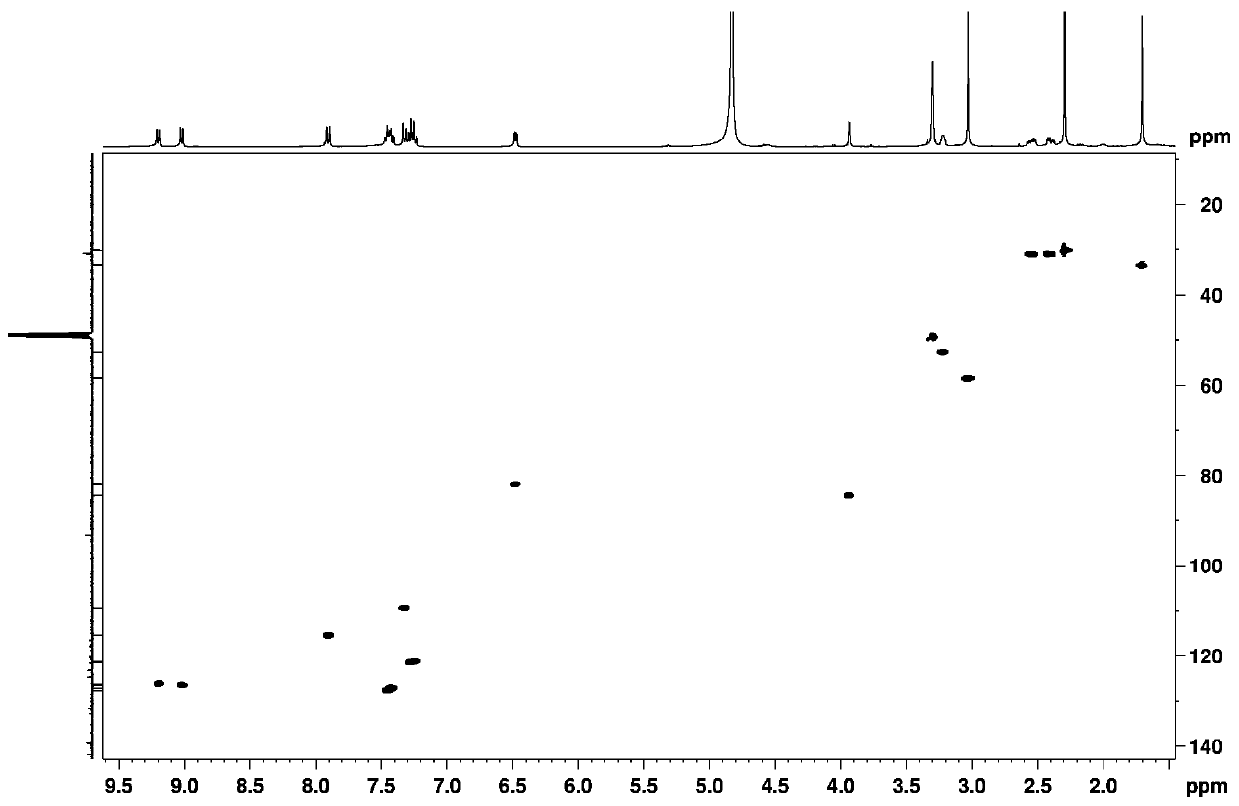
A 7-carbonyl staurosporine compound, its preparation method and its application in the preparation of anticancer drugs
A compound and carbonyl star technology, applied in the field of preparation of active compounds from secondary metabolites of actinomycetes, can solve problems such as poor selectivity, and achieve the effects of simple operation, easy cultivation, and easy production expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0050] 1. Seed solution
[0051]The above Streptomyces were inoculated into 500mL Erlenmeyer flasks containing 250mL of liquid medium, each bottle containing 250mL of Gao's No. 1 liquid medium, and cultured in a shaker at 28°C at 180rpm for 3 days to obtain seed liquid.
[0052] 2. Fermentation
[0053] Inoculate the above-mentioned seed liquid into the rice fermentation medium (made by the following weight components: rice 40g, 25% sea salt water 60mL) with the amount of inoculating 8mL per bottle, and stop the fermentation after 60 days of static culture at 28°C.
[0054] 3. Rough extraction
[0055] Soak each bottle of solid fermented product with about 200 mL of EA (ethyl acetate) overnight, filter through three layers of gauze to remove mycelia, collect the filtrate, and concentrate under reduced pressure to obtain a crude extract (oily extract).
[0056] 4. Separation and purification
[0057] The above crude extract was extracted three times with an equal volume of e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com