Soft capsule containing lubiprostone as well as preparation method thereof
A lubiprostone and soft capsule technology, applied in the field of drug preparation, can solve problems such as uneven bioavailability of lubiprostone, improve stability and bioavailability, reduce degradation, and bioavailability high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
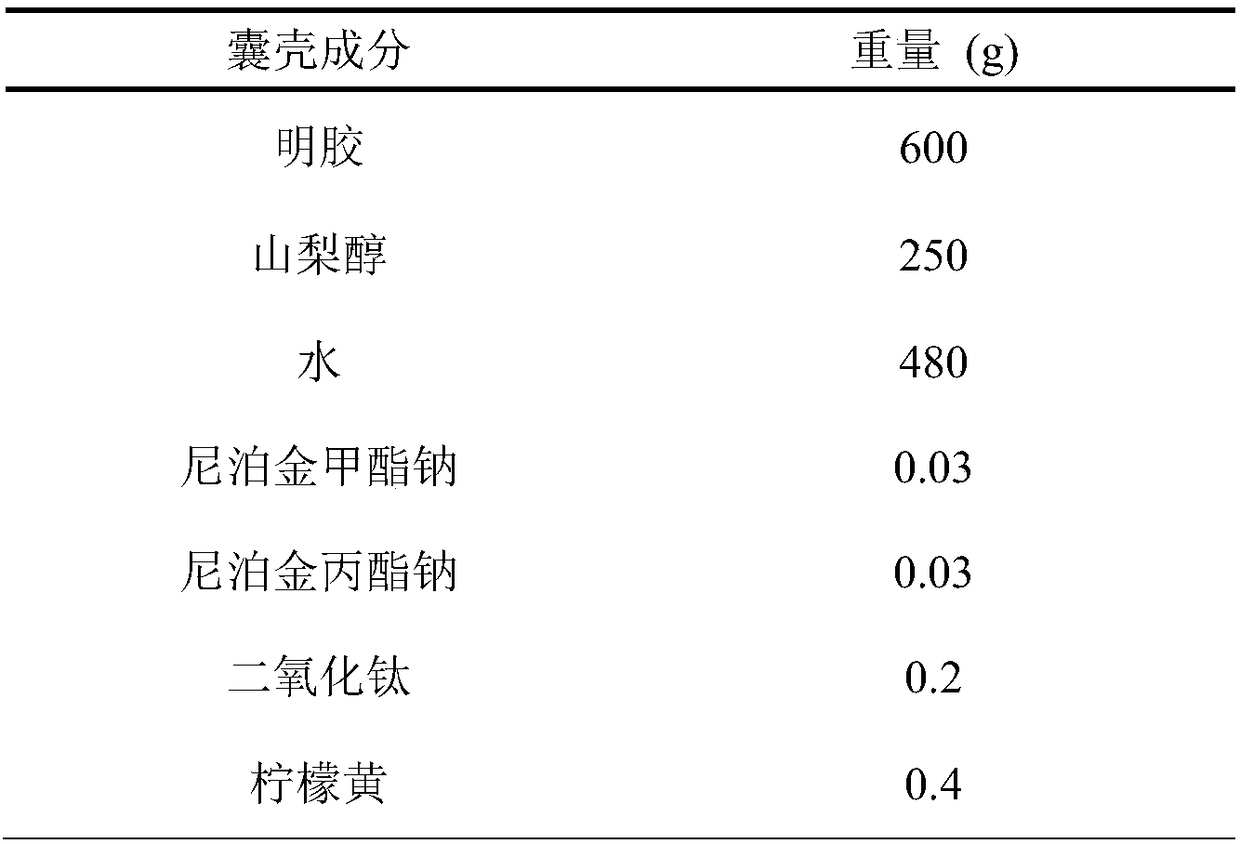
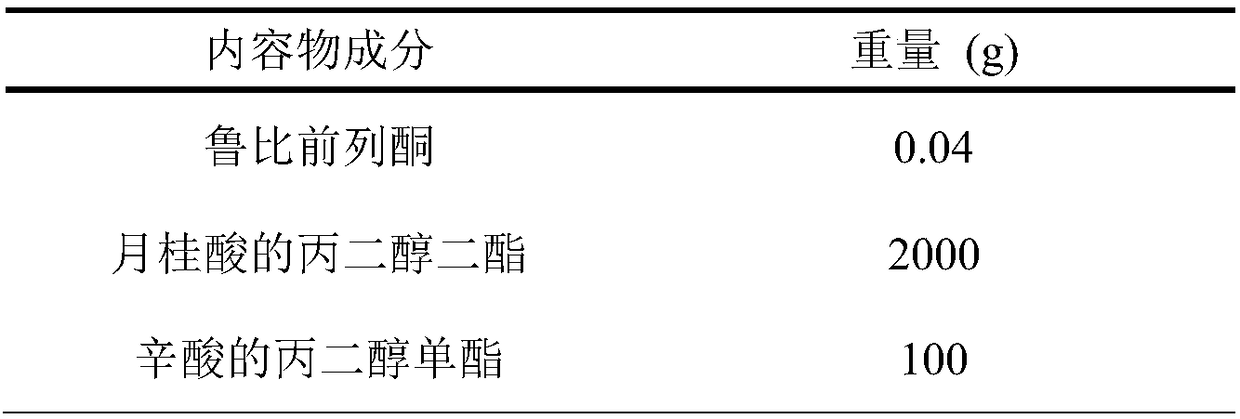
Embodiment 1
[0026] A preparation method of soft capsules containing lubiprostone, carried out as follows:
[0027] a. Take the raw materials of the soft capsule shell according to the proportion, heat them in the glue tank, mix them evenly, remove the bubbles under vacuum, take samples to measure the moisture and viscosity, and keep them warm at 50-70°C for later use after passing the inspection;
[0028] b. Heat and stir the solvent in a water bath at 70-80°C to form a uniform solution, cool to room temperature, add the main ingredient to dissolve completely, and set aside;
[0029] c. Determine the loading amount of the content according to the main drug content;
[0030] d. prepare soft capsules by compression method;
[0031] e. the prepared soft capsules are placed in a rotating drier for drying, and the oil stains on the surface are washed away with 95% ethanol, and then the soft capsules are dried in a drying room;
[0032] f. dry soft capsules are inspected and packaged.
[003...
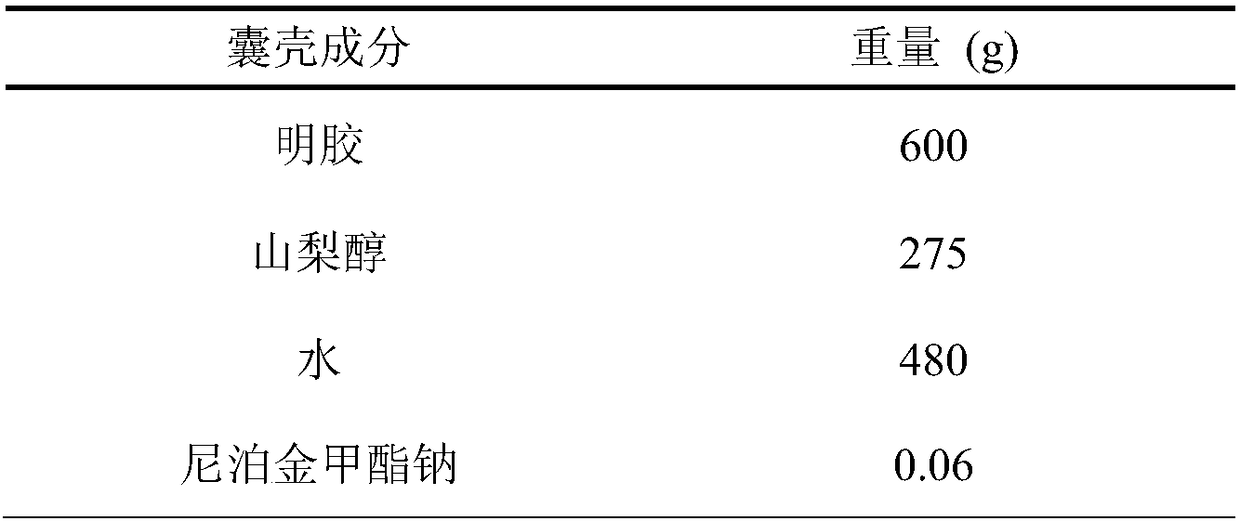
Embodiment 2
[0040] A preparation method of soft capsules containing lubiprostone, carried out as follows:
[0041] a. Take the raw materials of the soft capsule shell according to the proportion, heat them in the glue tank, mix them evenly, remove the bubbles under vacuum, take samples to measure the moisture and viscosity, and keep them warm at 50-70°C for later use after passing the inspection;
[0042] b. Heat and stir the solvent in a water bath at 70-80°C to form a uniform solution, cool to room temperature, add the main ingredient to dissolve completely, and set aside;
[0043] c. Determine the loading amount of the content according to the main drug content;
[0044] d. prepare soft capsules by compression method;
[0045] e. the prepared soft capsules are placed in a rotating drier for drying, and the oil stains on the surface are washed away with 95% ethanol, and then the soft capsules are dried in a drying room;
[0046] f. dry soft capsules are inspected and packaged.
[004...
Embodiment 3
[0055] A preparation method of soft capsules containing lubiprostone, carried out as follows:
[0056] a. Take the raw materials of the soft capsule shell according to the proportion, heat them in the glue tank, mix them evenly, remove the bubbles in vacuum, take samples to measure the moisture and viscosity, and keep them warm at 50-70°C for later use after passing the inspection;
[0057] b. Heat and stir the solvent in a water bath at 70-80°C to form a uniform solution, cool to room temperature, add the main ingredient to dissolve completely, and set aside;
[0058] c. Determine the loading amount of the content according to the main drug content;
[0059] d. prepare soft capsules by compression method;
[0060] e. the prepared soft capsules are placed in a rotating drier for drying, and the oil stains on the surface are washed away with 95% ethanol, and then the soft capsules are dried in a drying room;
[0061] f. dry soft capsules are inspected and packaged.
[0062] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com