Combination of c-met inhibitor with antibody molecule to pd-1 and uses thereof
A technology of PD-1 and antibody molecules, applied in the direction of antibody medical components, antibodies, drug combinations, etc., can solve the lack of MYPPY motifs and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0761] Embodiment 1: the pharmacokinetic analysis of flat dosing regimen
[0762] Based on pharmacokinetic (PK) modeling, flat doses are expected to provide exposure to patients at appropriate Cmin concentrations. More than 99.5% of patients will be above EC50 and more than 93% of patients will be above EC90. Steady-state mean Cmin predicted for the exemplary anti-PD-1 antibody molecule using 300 mg every three weeks (Q3W) or 400 mg every four weeks (Q4W) is expected to average above 20 ug / mL (maximum body weight, 150 kg).
[0763] Table 5 Exemplary PK Parameters Based on Flat Dosing Regimen
[0764]
[0765] The expected mean steady-state Cmin concentration of the exemplary anti-PD-1 antibody molecule observed with either dose / regimen (300mg q3w or 400mg q4w) would be about 77-fold higher than the EC50 (0.42ug / mL) and about 77 times higher than the EC90 8.6 times higher. Ex vivo efficacy is based on IL-2 changes in the SEB ex vivo assay.
[0766] For 300mg Q3W or 400mg...
Embodiment 2
[0769] Example 2: Phase Ib / II open-label, multi-center study of Capmatinib combined with anti-PD-1 antibody molecule ("Antibody A", detailed below) or antibody A single drug in the treatment of advanced hepatocellular carcinoma
[0770] antibody molecule A
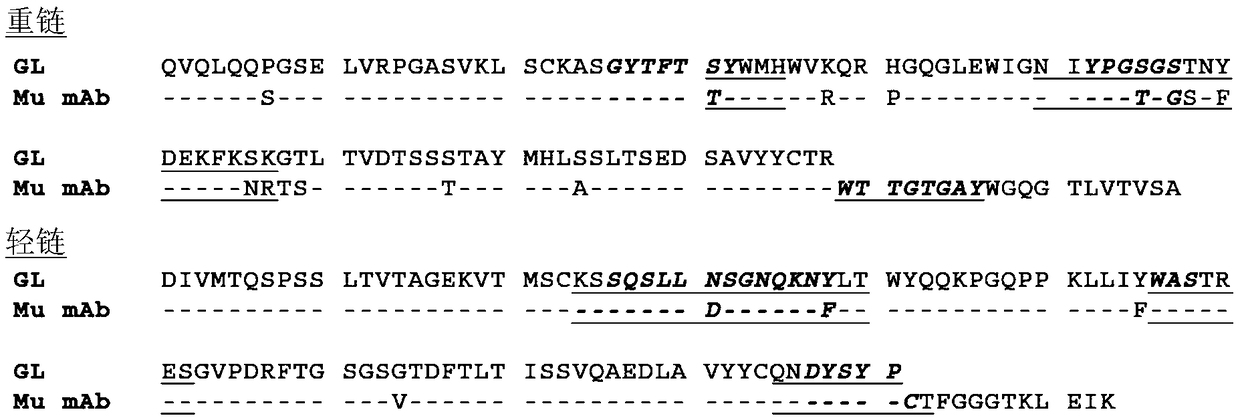
[0771] Antibody molecule A is a high affinity fully humanized anti-human PD-1 monoclonal antibody belonging to the IgG4 / κ isotype subclass. It is expressed in a Chinese hamster ovary cell line (CHO-C8TD) and consists of two heavy chains and two light chains. Both heavy chains of antibody molecule A contain oligosaccharide chains attached to the protein backbone at Asn294.
[0772] The amino acid sequences of the light chain (220 amino acids) and heavy chain (443 amino acids) deduced from the DNA sequences are shown in Figure 15 and Figure 16 .
[0773] Table 6 lists the expected disulfide bonds derived from the primary sequence.
[0774] Table 6 Expected disulfide bonds
[0775]
[0776] Based on the amino acid comp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| clearance rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com