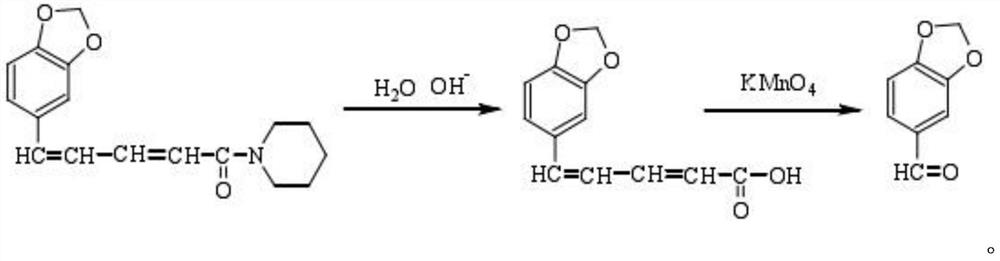
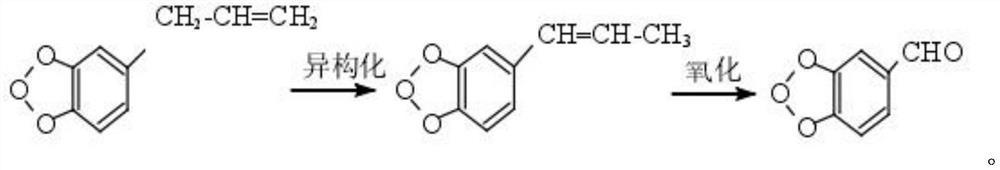
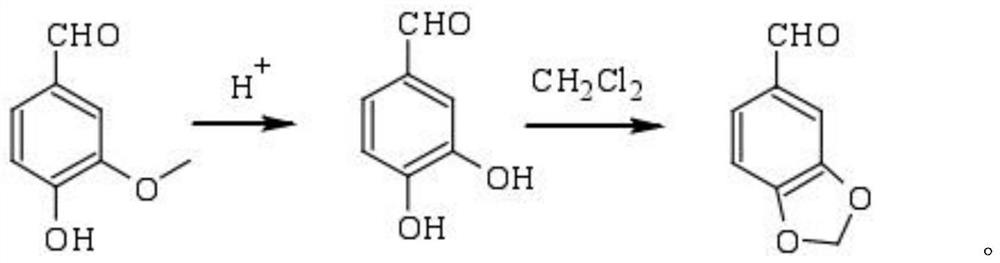
A kind of preparation method of piperonal
A technology of piperonal and piperonyl ring, which is applied in the field of synthesis of piperonal, can solve the problems of complex operation, difficulty in purchasing, and high price, and achieve the effects of low discharge of three wastes, simplified procedures, and easy disposal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Add 680g of polyphosphoric acid into a 2000ml three-necked flask, add 120ml of ethanol under stirring, heat up to 55°C, slowly add 50g (0.41mol) of piperonylcycline dropwise, and finish dripping in 0.5 hours, add 60g (0.43mol) of urotropine, and add After completion, start to heat up to 90-100°C, keep it warm for 3 hours, take a sample for liquid phase analysis, cool down to 60-70°C, add 700ml of water, stir for 3 hours, add ethyl acetate twice for extraction, 650ml each time, combine the organic phases, Add 400ml of saturated brine to wash, separate the organic phase, add 40g of anhydrous sodium sulfate to dry for 1 hour, filter, the filtrate is concentrated in ethyl acetate at 40°C, and the remaining liquid is distilled under reduced pressure by an oil pump to obtain 43g of piperonal with a purity of 99%.
[0053] 1H-NMR (300MHZ): (δ6.08(s, 2H); δ6.95(d, 1H); δ7.34(s, 1H); δ7.43(d, 1H); δ9.82(s, 1H).
Embodiment 2
[0055] Add 350g of polyphosphoric acid into a 1000ml three-necked bottle, add 100ml of ethanol while stirring, heat up to 55°C, slowly add 50g of peppercycline dropwise, and finish dropping in 0.5 hours, add 60g of urotropine, after the addition is complete, start to heat up to 70°C, Keep warm for 5 hours, sample liquid phase analysis, lower the temperature to 60-70°C, add 300ml of water, stir for 3 hours, add ethyl acetate for two extractions, 400ml each time, combine the organic phase, add 300ml of saturated saline to wash, and separate the organic phase. Phase, add anhydrous sodium sulfate 30g and dry for 1 hour, filter, the filtrate is concentrated ethyl acetate at 40 ℃, and the remaining liquid is distilled under reduced pressure by an oil pump to obtain 40g of piperonal with a purity of 99%.
Embodiment 3
[0057] Add 350g of polyphosphoric acid into a 1000ml three-necked bottle, add 100ml of ethanol while stirring, heat up to 55°C, slowly add 50g of peppercycline dropwise, and finish dropping in 0.5 hours, add 60g of urotropine, after the addition is complete, start to heat up to 70°C, Insulated for 5 hours, sampled for liquid phase analysis, concentrated ethanol under reduced pressure, and distilled under reduced pressure by an oil pump to obtain 32 g of piperonal with a purity of 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com