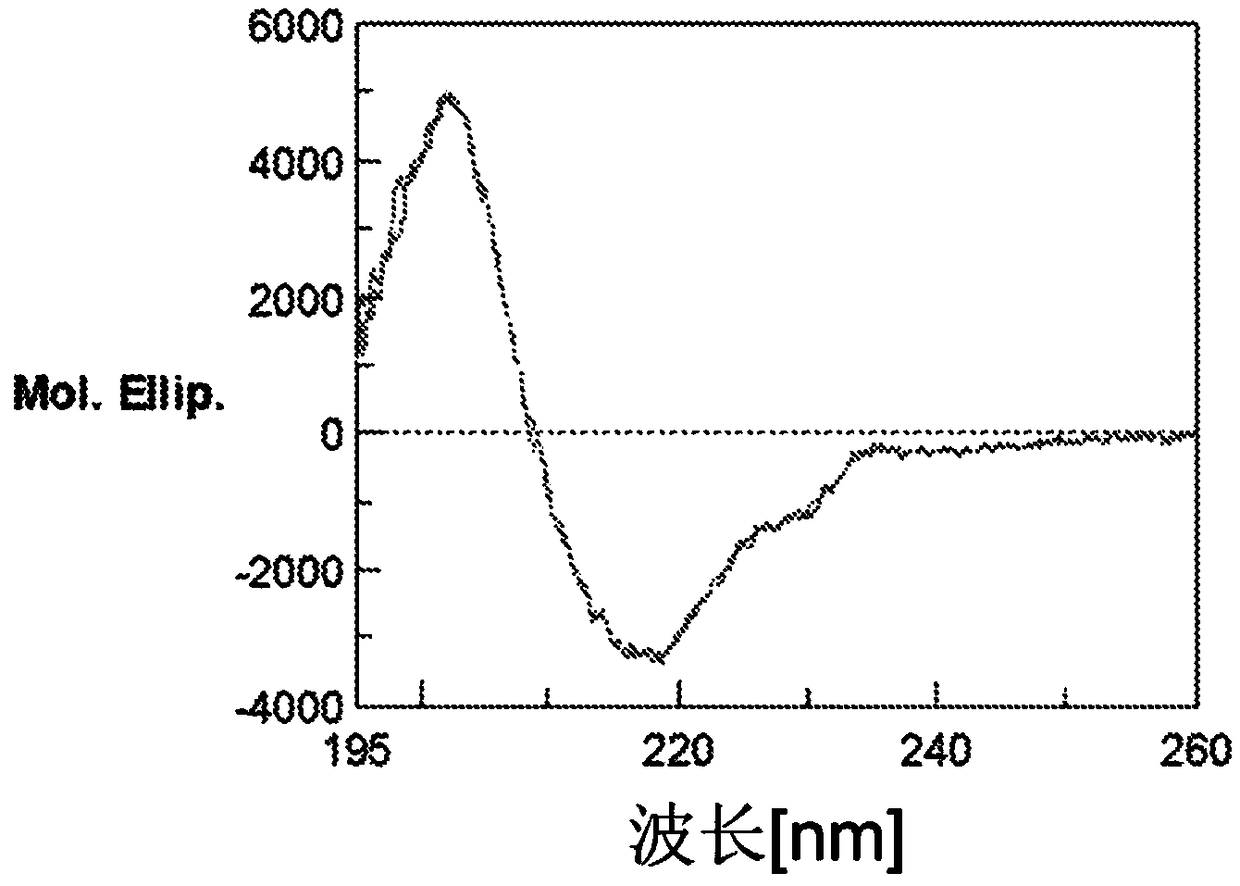
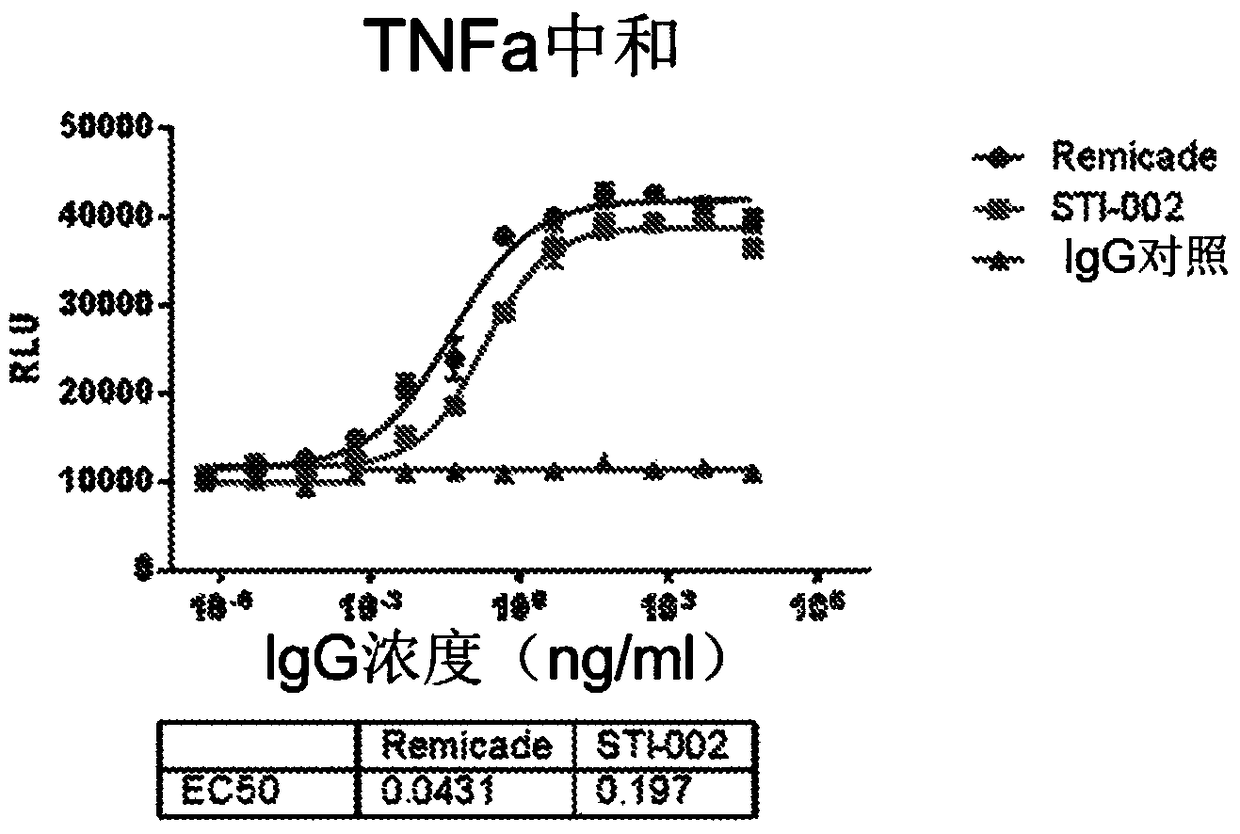
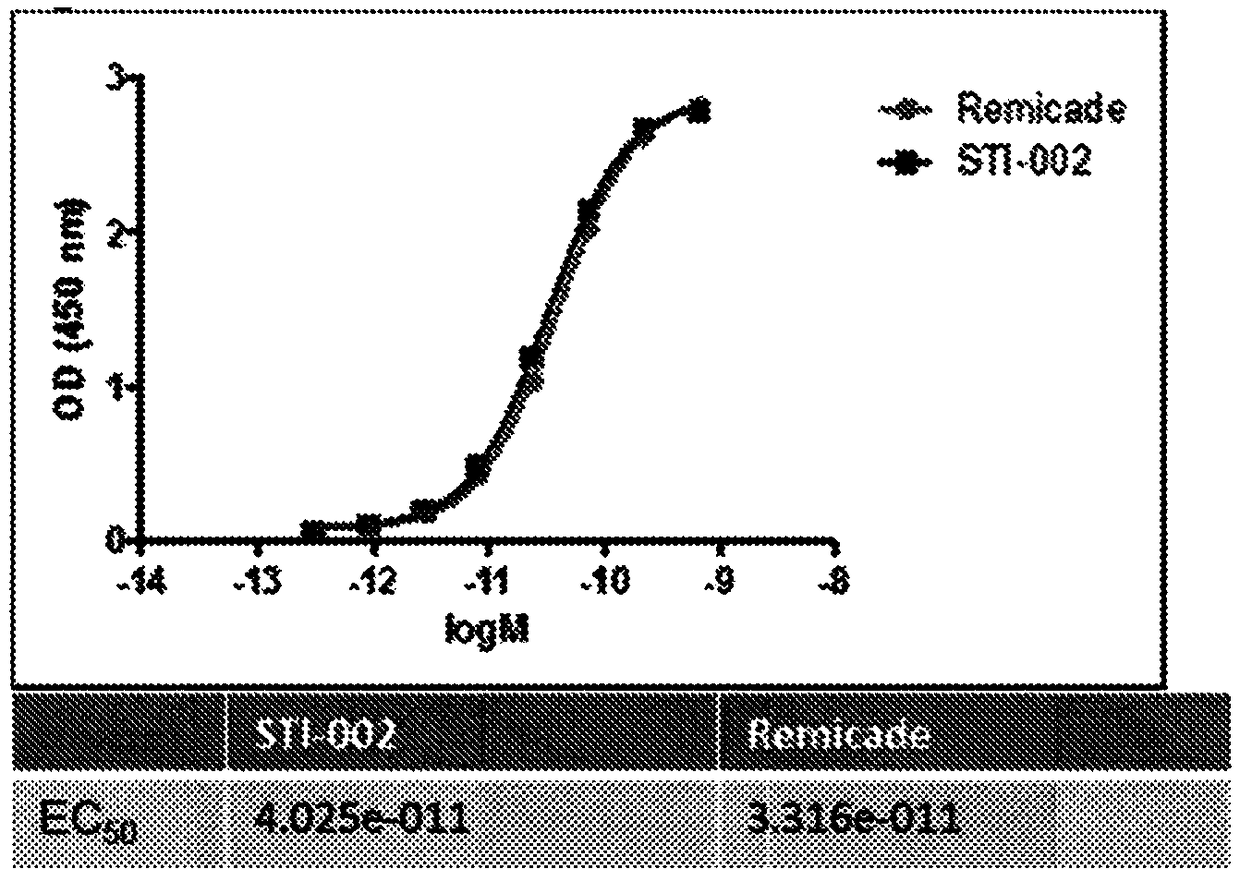
Improved safety and efficacy with CHO cell glycosylated chimeric antibody to TNF
A glycosylation, antibody technology, applied in the direction of antibodies, chemical instruments and methods, anti-inflammatory agents, etc., can solve problems such as adverse reactions of patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0013] The present invention is based at least in part on the therapeutic advantages of producing anti-TNF antibodies in Chinese Hamster Ovary (CHO) cells. STI002 is an anti-TNF antibody that is produced in CHO cells and has the amino acid sequence of infliximab. Structurally, infliximab has a light chain comprising the amino acid sequence shown in SEQ ID NO:1, and a heavy chain comprising the amino acid sequence shown in SEQ ID NO:2. The amino acid sequences of the light and heavy chains of Infliximab are described below:
[0014] Asp Ile Leu Leu Thr Gln Ser Pro Ala Ile Leu Ser Val Ser Pro Gly GluArg Val Ser Phe Ser Cys Arg Ala Ser Gln Phe Val Gly Ser Ser Ile His Trp TyrGln Gln Arg Thr Asn Gly Ser Pro Arg Leu Leu Ile Lys Tyr Ala Ser Glu Ser MetSer Gly Ile Pro Ser Arg Phe Ser Gly Ser Gly Ser Gly Thr Asp Phe Thr Leu SerIle Asn Thr Val Glu Ser Glu Asp Ile Ala Asp Tyr Tyr Cys Gln Gln Ser His SerTrp Pro Phe Thr Phe Gly Ser Gly Thr Asn Leu Glu Val Lys (SEQ ID NO: 1)
[0015] Glu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com