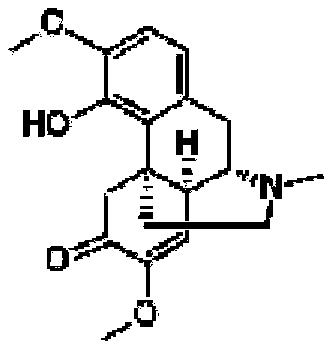
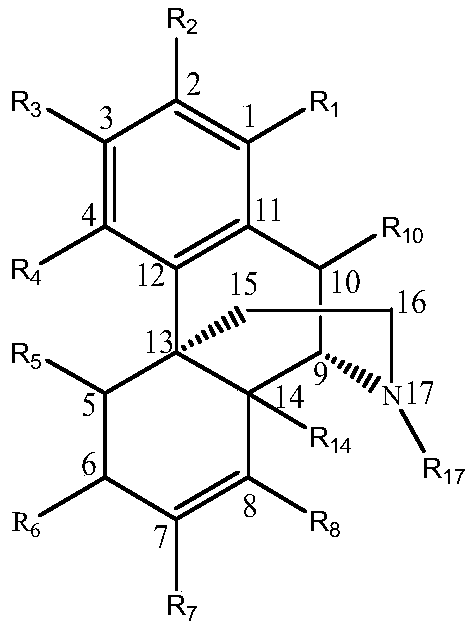
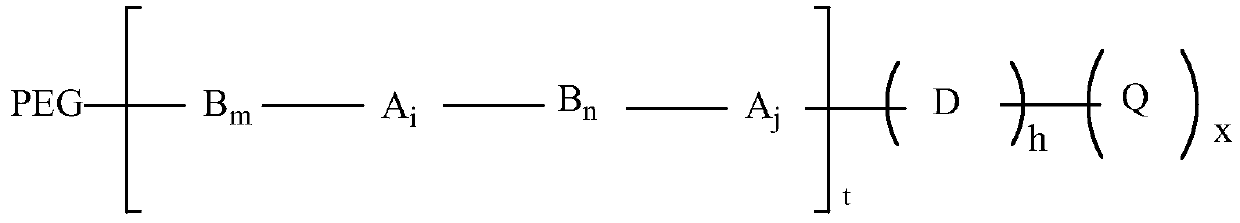A peg modification of sinomenine and its derivatives and its preparation
A technology of sinomenine and its derivatives, which is applied in the field of PEG modification and its drug combination, and in the field of medicine, and can solve problems such as prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0094] Example 1: PEG 20k -[-LYS-(-CO-CH 2 CH 2 -CO-O-Sinomenine) 2 ] 2 PEG20K-cyan
[0095]
[0096] (1) Synthesis of sinomenine-4-succinic acid monoester (A1)
[0097] Add sinomenine (3.3g, 10mmol), succinic anhydride (2g, 20mmol), 4-dimethylaminopyridine (DMAP, 300mg, 2.5mmol), dicyclohexylcarbodiimide (DCC, 2.1g, 10mmol) into 100ml In the single-necked bottle, 60ml of dichloromethane was added under the protection of nitrogen, the temperature was raised to 50° C. and the reaction was stirred for 48 hours, and the reaction was detected by thin-layer chromatography. The reaction solution was concentrated, and the residual solution was added to the ice-water mixture with stirring, stirred for 30 min, crystallized at 0°C overnight, filtered, the filter cake was washed with ice water, and the filter cake was dried to obtain 4.9 g of light yellow solid.
[0098] (2) Synthesis of Dilysyl-Polyethylene Glycol (A2)
[0099] In a one-mouth bottle, add successively, bisamin...
Embodiment 2
[0103] Example 2: mPEG 5k -CO-NHCH 2 -CO-O-Sinomenine Experimentally called PEG5K-cyan
[0104]
[0105] (1) Synthesis of sinomenine-4-glycine monoester (B1)
[0106] Sinomenine (3.3g, 10mmol), N-tert-butoxycarbonylglycine (8.7g, 50mmol), 4-dimethylaminopyridine (DMAP, 300mg, 2.5mmol), dicyclohexylcarbodiimide (DCC, 3.1 g (15 mmol) was added to a 250 ml single-necked bottle, and 100 ml of dichloromethane was added under nitrogen protection, and the reaction was stirred at room temperature for 48 hours, and the reaction was detected by thin-layer chromatography. Filtrate, concentrate the reaction solution, add 100ml of diethyl ether, stir for 30min, filter, and vacuum-dry to obtain 11g of white sinomenine-4-(N-tert-butoxycarbonyl)glycine monoester solid.
[0107] Add 10 g of sinomenine-4-(N-tert-butoxycarbonyl)glycine monoester from the previous step into a 250ml single-necked bottle, add 100ml of dichloromethane to dissolve, add 30ml of trifluoroacetic acid after complet...
Embodiment 3
[0110] Example 3: mPEG-6-sinomenine ether oxalate (sinomenine 6-(-3,6,9,12,15,18,21-heptaoxodocosane) ether oxalate experiment mPEG-6-cyan
[0111]
[0112] (1) Synthesis of 4-MEM-6-OH-sinomenine
[0113] Join sinomenine (10g, 30mmol) in the 500ml round bottom flask, add 300ml dichloromethane, then add DIPEA (23.5ml, 18.4g, 180mmol) and stir, add MEMCl (13.7ml, 14.9g, 120mmol) under ice-water bath ) after 30 min of dropwise addition, the temperature was naturally raised to room temperature, and the reaction was carried out overnight. The reaction solution was washed with saturated sodium chloride solution, dried over anhydrous sodium sulfate for 1 hour, filtered, and concentrated to obtain 12.01 g of 4-MEM-sinomenine product.
[0114]4-MEM-sinomenine, (12.01g, 28.8mmol) was added to a 500ml round bottom flask, then a mixed solution of 120ml acetonitrile and 120ml acetic acid was added, the temperature was lowered to -15°C, and tetramethylammonium triacetoxyborohydride ( 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com