A kind of synthetic method of efavirenz key intermediate
A synthetic method, the technology of efavirenz, applied in the field of preparation of efavirenz, can solve the problems of high price and dosage of organic ligands, unsuitable for industrial production, harsh reaction conditions, etc., and achieve simple route and excellent reaction yield , the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
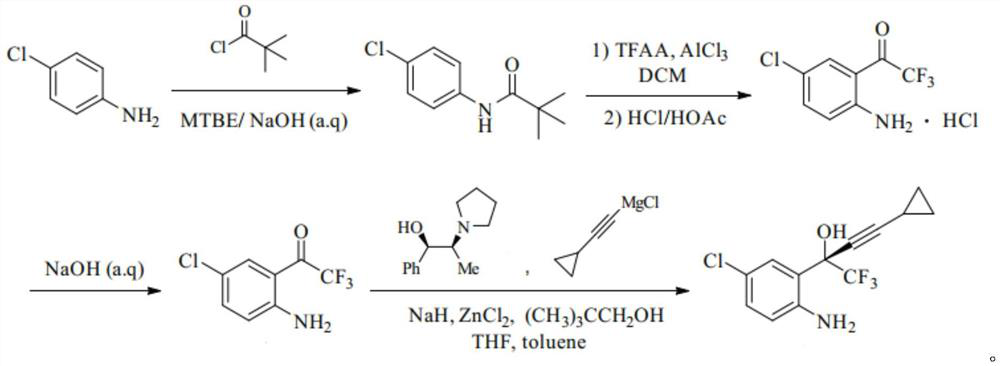
[0017] A kind of synthetic method of efavirenz key intermediate, comprises the following steps:
[0018] S1: Add 25.50g of p-chloroaniline to a mixture of 70ml of methyl tert-butyl ether and 24ml of sodium hydroxide solution (10mol / L), cool down to 10°C, slowly add 25.60g of pivaloyl chloride dropwise, drop for 40min After completion, keep stirring at 10-15°C for 2 hours, then rise to room temperature and stir for 4 hours. Cooled to 0°C, white crystals precipitated, filtered with suction, washed the filter cake with water, and dried under reduced pressure at 55°C for 5 hours to obtain 41.27g of white solid N-(4-chlorophenyl)-2,2-dimethylpropanamide ;
[0019] S2: Add 60.10g of anhydrous aluminum trichloride to 300ml of dichloromethane, lower the temperature to -20°C, slowly add 44.10g of TFAA dropwise, control the temperature at -23~-20°C, and finish dropping in 1h. Insulation reaction 2h. Add 38.11 g of N-(4-chlorophenyl)-2,2-dimethylpropanamide in 3 batches at -25°C, comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com