Chromane bridged-ring isoindolinone and preparation method thereof
A technology of isoindolinone and bridged ring, which is applied in the field of chroman bridged ring isoindolinone and its preparation, and achieves the effects of mild reaction conditions, high yield and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
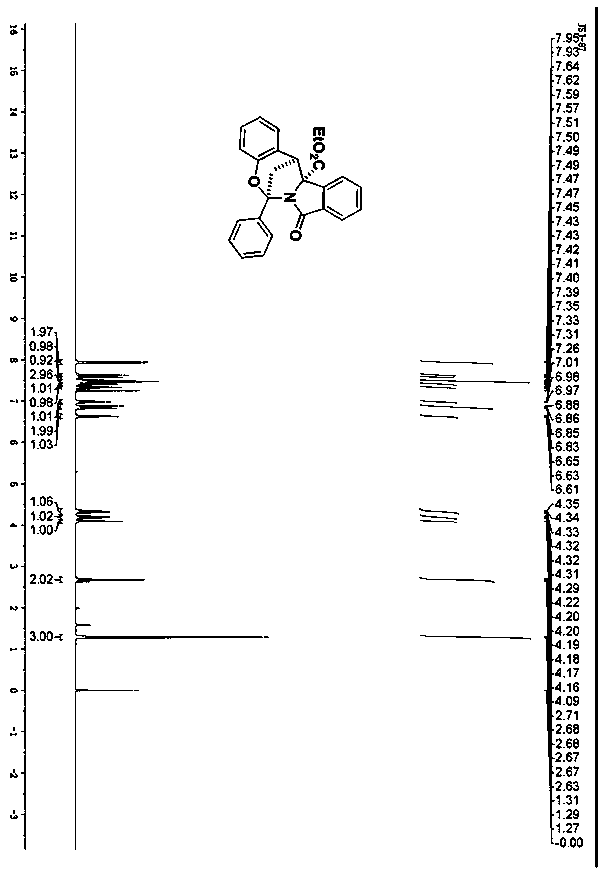
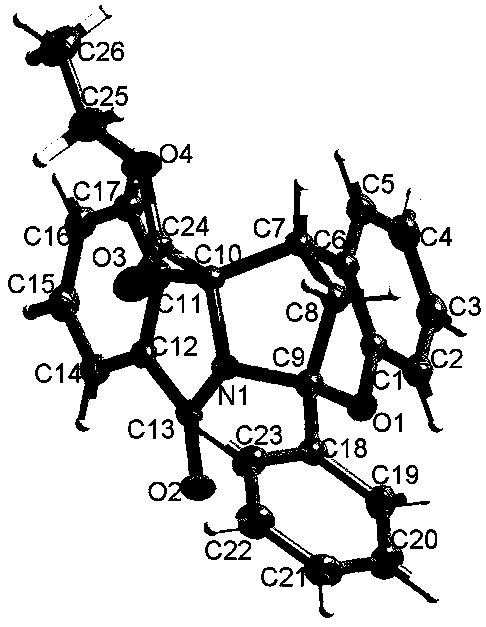
[0025] Preparation of chroman-bridged isoindolinone 3a: Into a 10mL hard glass reaction tube, add isoindolinone 1a (0.22mmol, 45.2mg), o-hydroxychalcone 2a (0.20mmol, 44.9mg), for Toluenesulfonic acid (6.9 mg, 0.04 mmol) was added, then 1 mL of acetonitrile was added, and the mixture was stirred at 35° C. for 24 h. After the reaction was complete, it was filtered, and the filter cake was the target compound 3a (white solid, yield 97%).
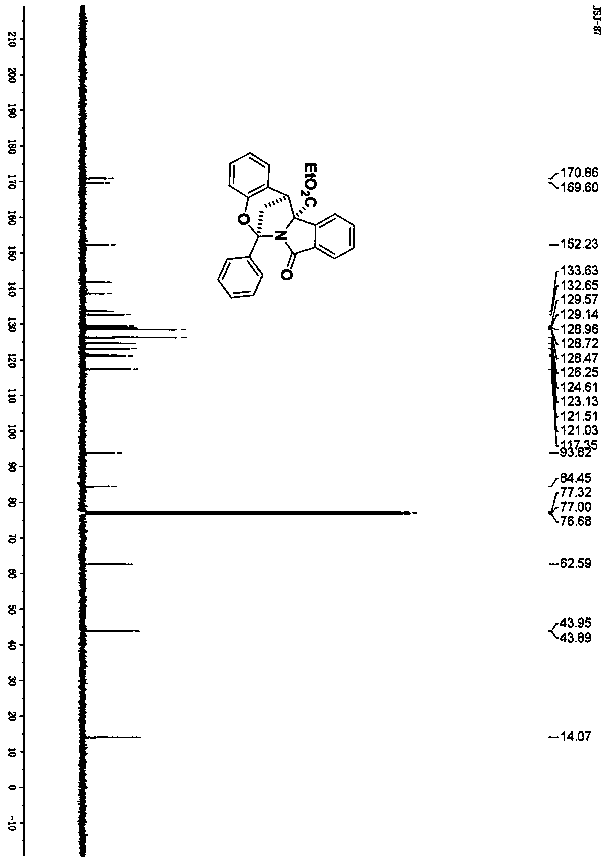
[0026] The obtained compound 3a proton nuclear magnetic resonance spectrum, carbon spectrum, infrared and high resolution mass spectrometry data are as follows:
[0027] 1 H NMR (400MHz, CDCl 3 ), δ7.94(d, J=8.0Hz, 2H), 7.63(d, J=8.0Hz, 1H), 7.58(d, J=8.0Hz, 1H), 7.51-7.45(m, 3H), 7.43 -7.39(m, 1H), 7.33(t, J=8.0Hz, 1H), 6.98(t, J=8.0Hz, 1H), 6.87(d, J=8.0Hz, 1H), 6.84(d, J= 8.0Hz,1H),6.63(t,J=8.0Hz,1H),4.35-4.29(m,1H),4.22-4.16(m,1H),4.09(s,1H),2.71-2.63(m,2H ),1.29(t,J=8.0Hz,3H); 13 CNMR (100MHz, CDCl 3 )δ 170.9, 169.6, 152.2, 141.8, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com