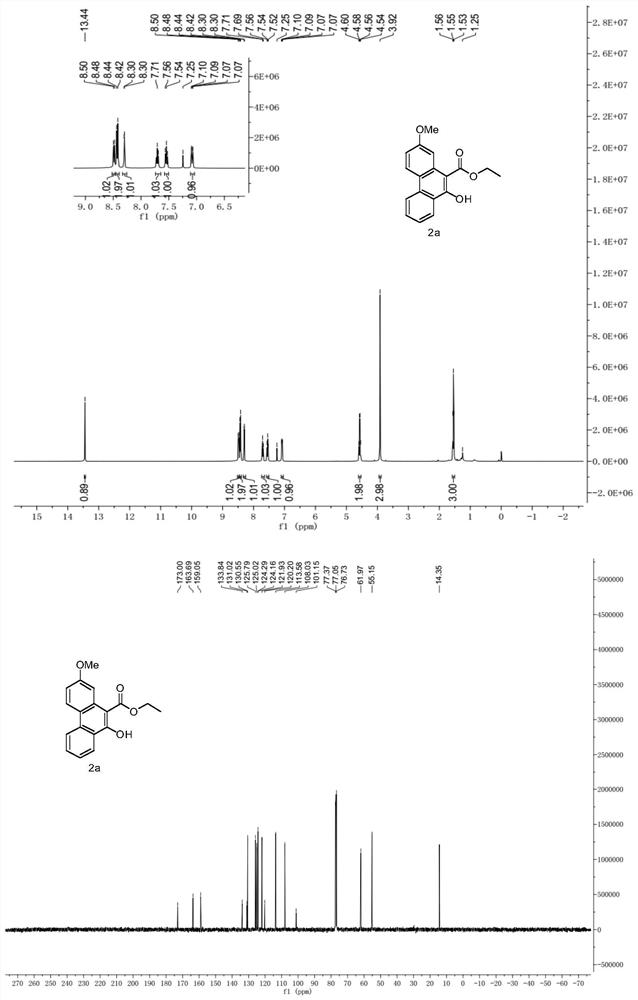
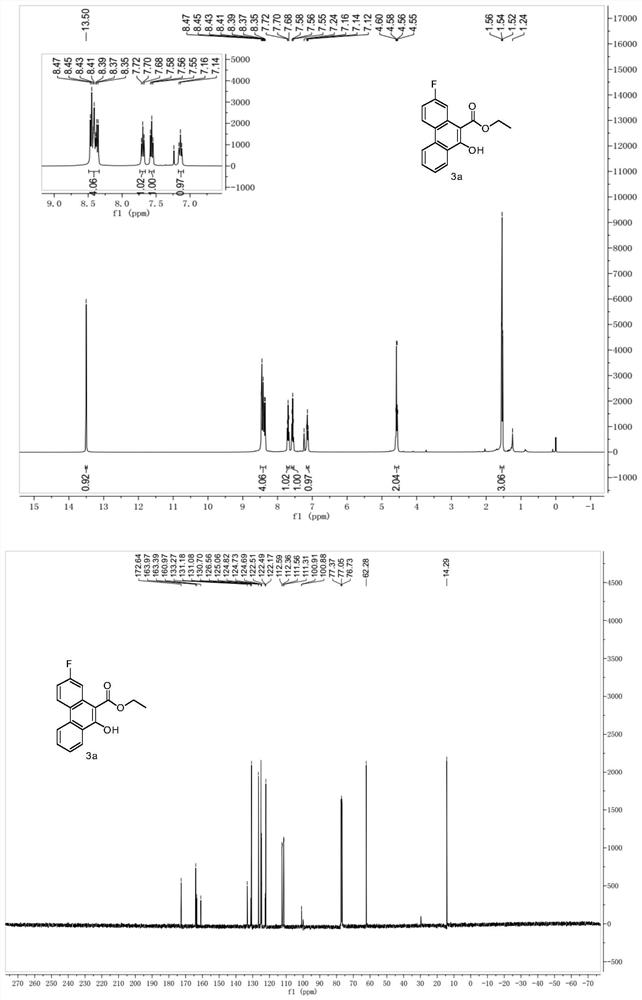
A kind of multi-substituted 10-hydroxyphenanthrene and its derivatives and its synthesis method
A synthesis method and technology of hydroxyphenanthrene are applied in pharmaceutical chemical intermediates and related chemical fields, and can solve the problems of difficult preparation of substrates, many reaction steps, environmental pollution, etc., and achieve easy products, short synthesis routes, and wide substrates. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
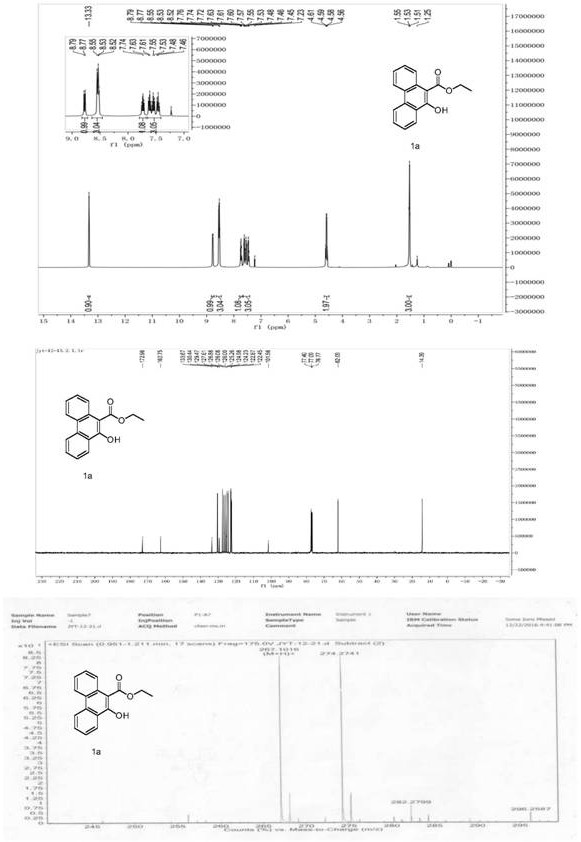
[0050] Example 1: Synthesis of ethyl 10-hydroxyphenanthrene-9-carboxylate (1a)
[0051]
[0052] The method for synthetic formula (1) compound comprises the steps:
[0053] Step 1: Add tetrakis(triphenylphosphine)palladium (0.3mmol, 346.7mg) and anhydrous potassium carbonate (9mmol, 1.24g) into the three-necked flask. After sealing, exhaust the air in the bottle and fill it with nitrogen. Repeat once. Then add 2'-bromoacetophenone (3 mmol, 405 μL), a mixture of phenylboronic acid (3.6 mmol, 435 mg) dissolved in 3 mL of ethanol, and 12 mL of toluene saturated with nitrogen into the three-necked flask. The reaction mixture was refluxed at 110° C. for 11 hours to obtain the intermediate product biphenylphenone. almost completely converted. Separation and purification by silica gel column (petroleum ether: ethyl acetate = 100:1).
[0054] Step 2: Add 10 mL of anhydrous tetrahydrofuran to a three-necked flask, then add sodium hydride (dispersed in kerosene, content: 60%, 8.4...
Embodiment 2
[0060] Example 2: Synthesis of ethyl 10-hydroxyphenanthrene-9-carboxylate (1a)
[0061]
[0062] The method for synthesizing the compound of formula (1) is as described in Example 1.
[0063] The method for synthetic formula (2) compound comprises the steps:
[0064] Add formula (1) compound ethyl 3-([1,1'-biphenyl]-2-yl)-3-oxopropanoate (0.25mmol, 67mg), N-bromosuccinimide (0.1mmol , 17.8mg), tert-butyl hydroperoxide (0.875mmol, 78.86mg), sodium dihydrogen phosphate dihydrate (0.25mmol, 39mg), tetrahydrofuran 2mL, sealed, and stirred at 90°C for 15 hours. After cooling to room temperature and removing the solvent under reduced pressure, 57.2 mg of the compound ethyl 10-hydroxyphenanthrene-9-carboxylate (1a) of formula (2) was obtained by separation and purification by column chromatography (petroleum ether as the eluent), with a yield of 86%.
Embodiment 3
[0065] Example 3: Synthesis of ethyl 10-hydroxyphenanthrene-9-carboxylate (1a)
[0066]
[0067] The method for synthesizing the compound of formula (1) is as described in Example 1.
[0068] The method for synthetic formula (2) compound comprises the steps:
[0069] Add formula (1) compound ethyl 3-([1,1'-biphenyl]-2-yl)-3-oxopropanoate (0.25mmol, 67mg), N-bromosuccinimide (0.1mmol , 17.8mg), bromoethylamine hydrobromide (0.1mmol, 20.5mg), tert-butyl hydroperoxide (0.875mmol, 78.86mg), sodium dihydrogen phosphate dihydrate (0.25mmol, 39mg), tetrahydrofuran 2mL , sealed, and stirred at 80°C for 15 hours. After cooling to room temperature and removing the solvent under reduced pressure, separation and purification by column chromatography (petroleum ether as the eluent) gave 61.9 mg of the compound ethyl 10-hydroxyphenanthrene-9-carboxylate (1a) of formula (2), with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com