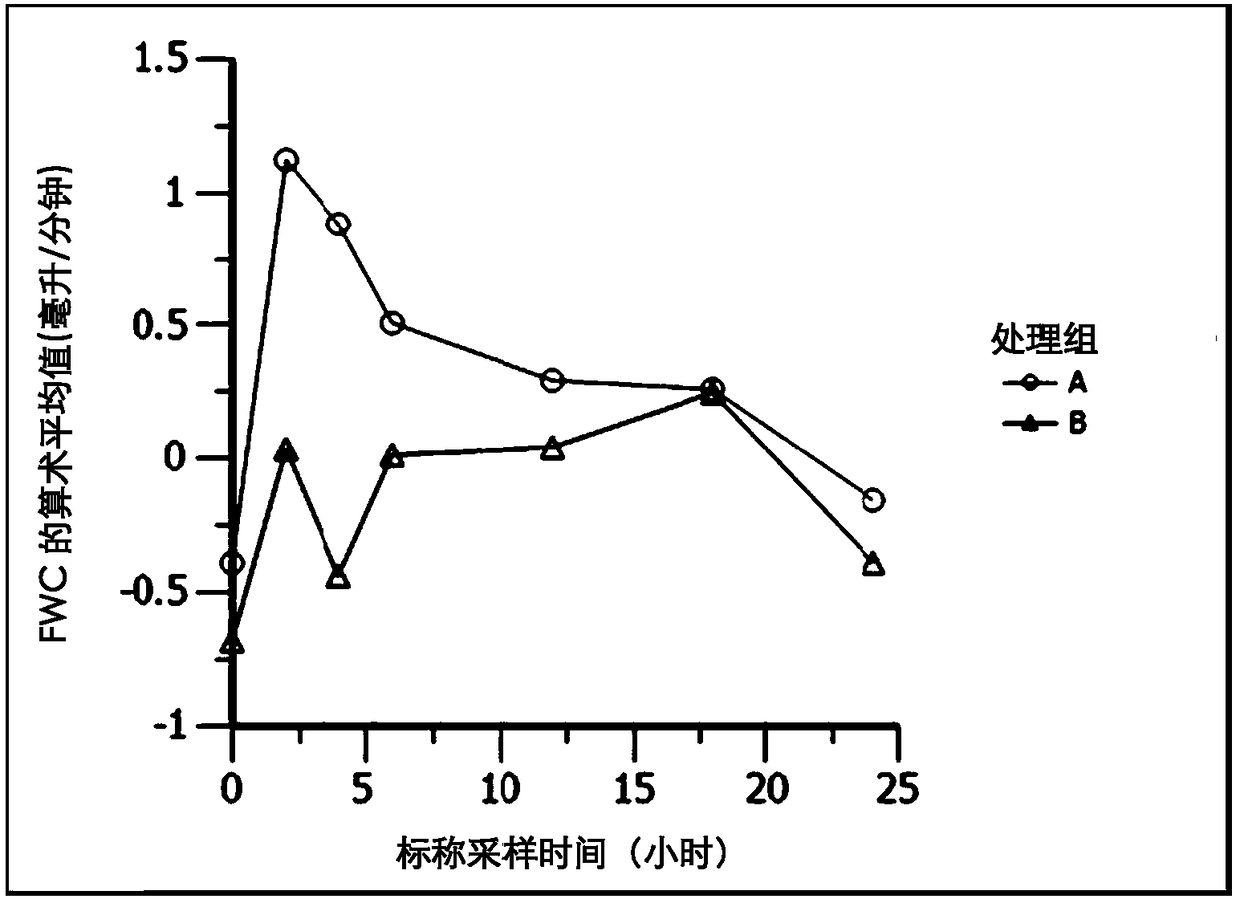
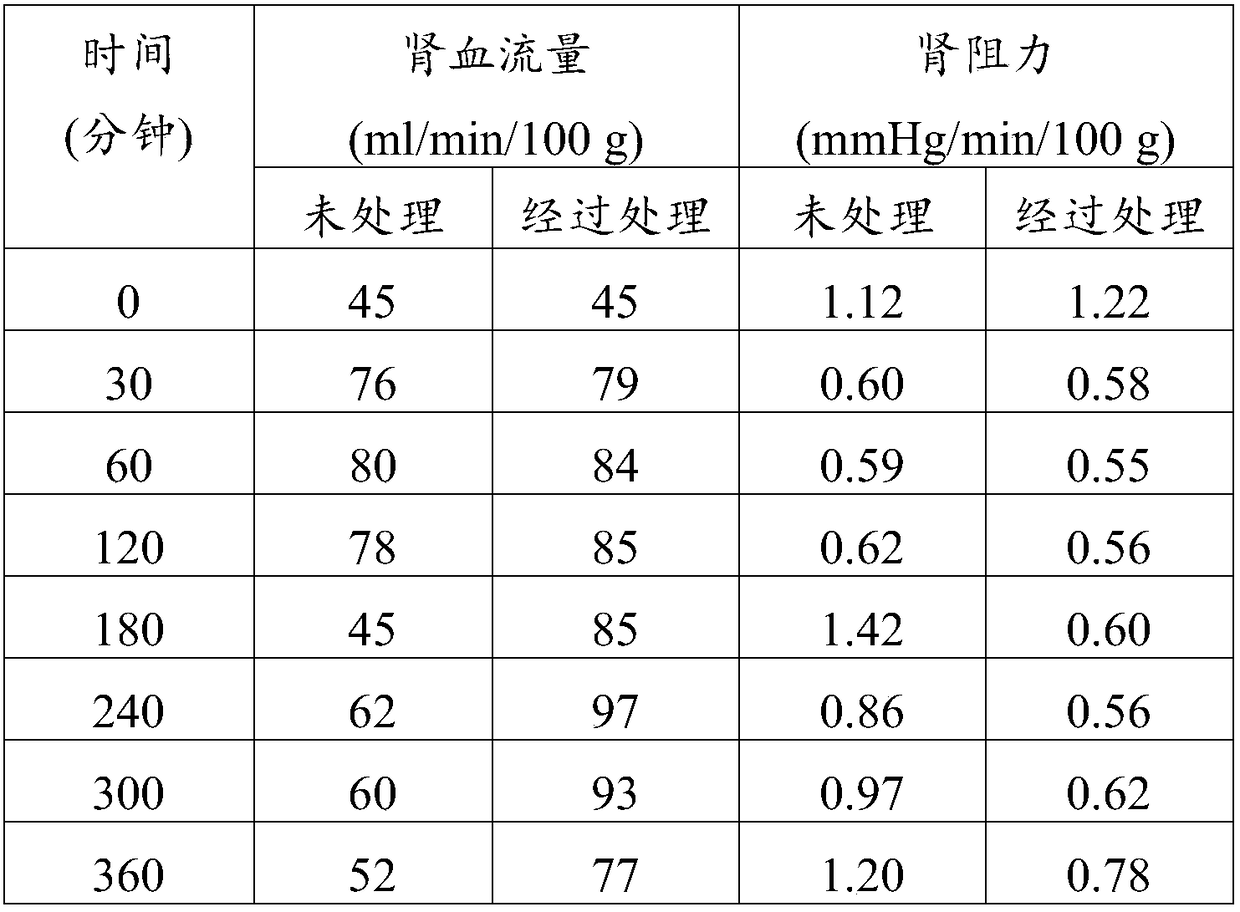
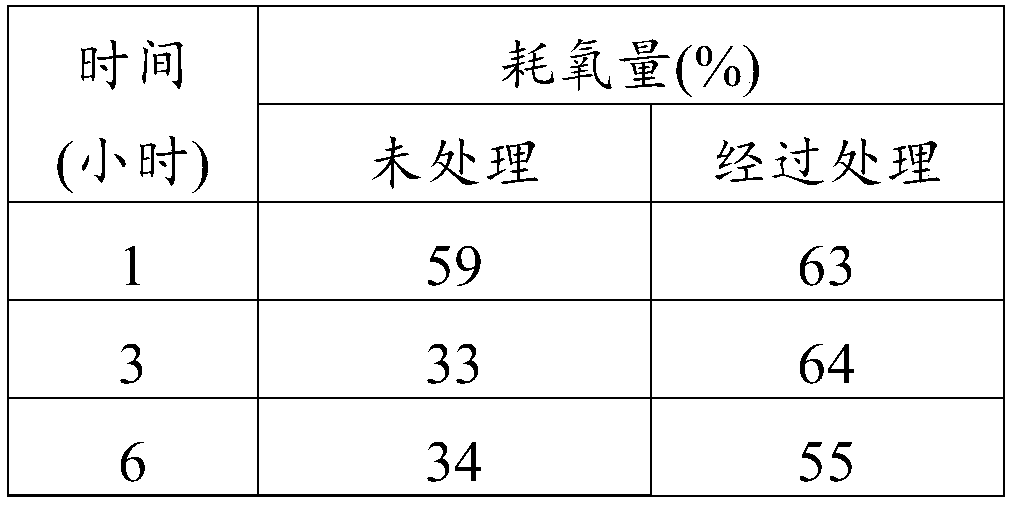
Ambrisentan for use in the treatment of acute renal failure
Ambrisentan, a technology for acute kidney injury, is used in the field of therapeutic and preventive formulations to address decreased renal blood flow, poor response to diuretic therapy, and low urinary and sodium excretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0116] Embodiment 1: Preparation of ambersentan solution:
[0117] A liquid composition was prepared containing 0.5 mg of ambrisentan per ml of solvent. First, 4.60 g of sodium acid phosphate monohydrate (NaH 2 PO 4 ·H 2 O), 4.73g anhydrous disodium hydrogen phosphate (Na 2 HPO 4 ) and 4.80 g of sodium chloride were dissolved in 1000 ml of sterile distilled water (sufficient) to obtain a solution with a pH value of 7.4. The resulting solution was heated to 50°C. Subsequently, 0.50 g of ambrisentan was added and the solution was stirred until the ambrisentan was completely dissolved. The resulting solution is cooled and sterilized by passing the solution through a suitable filter. The resulting liquid composition is in accordance with the invention and contains 0.5 mg / ml of ambrisentan. Analysis of the resulting liquid composition showed that ambrisentan remained stable and no decomposition was observed.
[0118] stability test
[0119] The liquid composition of Exam...
Embodiment 2
[0120] Embodiment 2: Preparation of Ambersentan preparation:
[0121] A liquid composition containing 10 mg of ambrisentan per ml of solvent was prepared. First, 4.00 g of polysorbate 20, 10.00 g of sodium ascorbate, 1.50 g of sodium chloride, 1.10 g of EDTA, and 9.20 g of anhydrous disodium hydrogen phosphate were dissolved in 1000 ml of sterile distilled water (sufficient) to obtain pH of the solution. The resulting solution was heated to 50°C. Subsequently, 10.00 g of Ambrisentan was added and the solution was stirred until Ambrisentan was completely dissolved. The resulting solution is cooled and sterilized by passing the solution through a suitable filter. The resulting liquid composition is in accordance with the invention and contains 10 mg / ml of ambrisentan.
Embodiment 3
[0122] Example 3: Treatment of porcine kidney with a liquid composition containing ambrisentan following ischemic injury
[0123] All experiments were performed in accordance with the Home Office animals (Scientific Procedures) Act 1986. Ten kidneys were removed from five Landrace crossbred female pigs weighing approximately 50 kg. Two pairs of kidneys were subjected to warm ischemic injury for 20 minutes, then flushed with 500 ml of UW solution at 4°C and stored on ice for 18 hours. After 18 hours of refrigeration, the kidneys were prepared and flushed with 100 ml of Ringer's solution (4° C.) with or without the liquid composition of Example 1 diluted to an ambrisentan concentration of 150 μg / l. They were then left at room temperature for 20 minutes. The remaining three pairs of kidneys were removed after 10 minutes of warm ischemia and stored on ice for 6 hours. They were prepared and rinsed as previously described, then left at room temperature for 10 min before reperf...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap